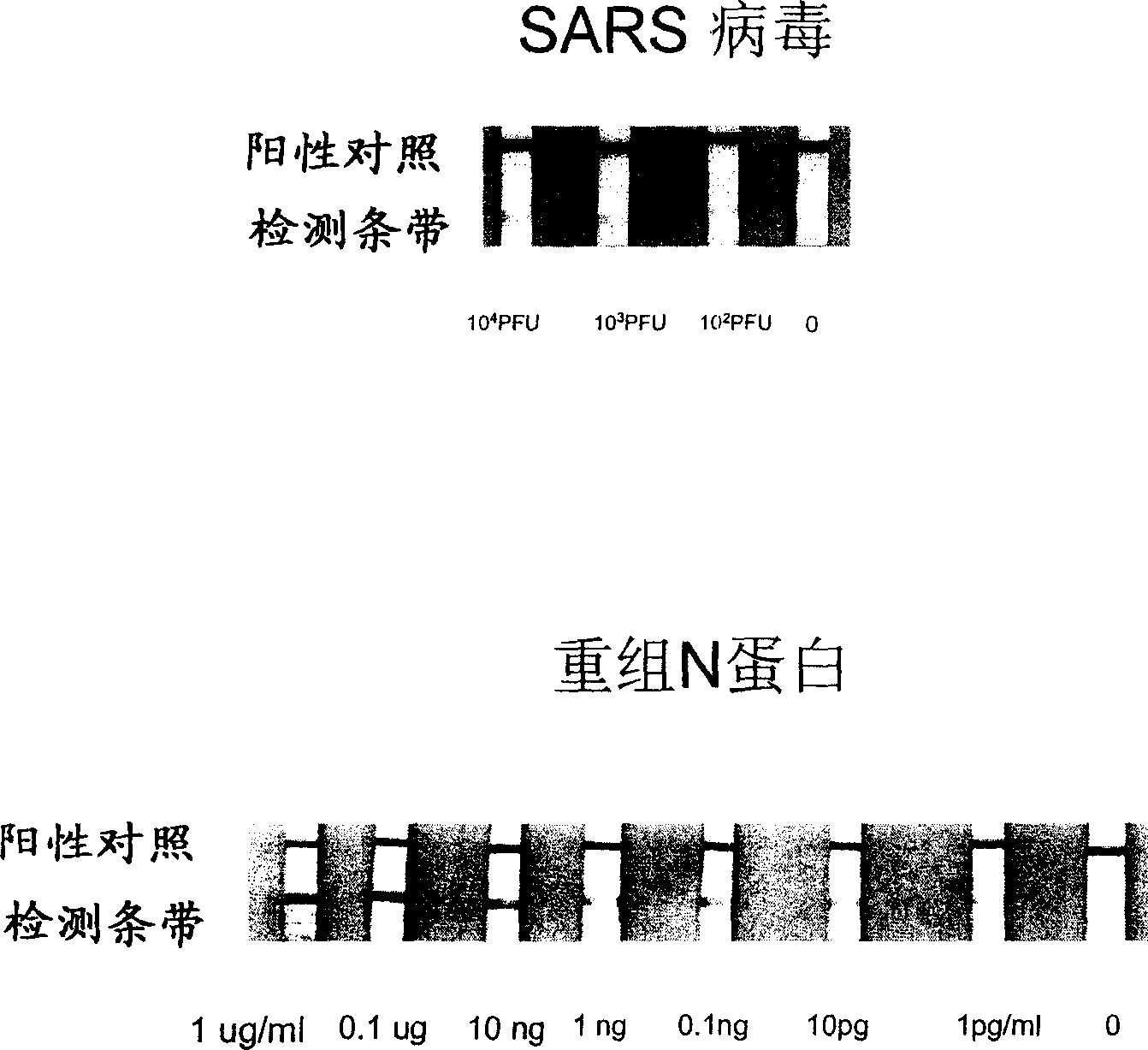
Antibody pointed at SARS coronavirus N protein antigen and its use in detecting SARS coronavirus or its antigen
A coronavirus, protein antigen technology, applied in the field of antibodies against SARS coronavirus N protein antigen and its application in SARS coronavirus or its antigen detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1, N protein gene synthesis:
[0064] The full-length nucleic acid sequence of the N protein is 1269bp, and the full-length protein sequence has a total of 422 amino acids. Because the SARS virus is the pathogen of a serious life-threatening infectious disease transmitted through the air, it is safe and simple to artificially synthesize it from safety considerations. According to the SARS coronavirus nucleocapsid protein (NP) gene complete coding region nucleotide sequence (Locus: AY307165 Protein_id=" AAP49024.1 " published in GenBank on June 19, 2003) synthetic whole coding region sequence, this sequence is shown in SEQ ID NO: 1, the deduced amino acid sequence of which is shown in SEQ ID No: 26.
[0065] The strategy for the synthesis of N protein-coding genes is as follows:
[0066] 1 3 5 7 9 11 13 15 17 19
[0067] 5' - - - - - - - - - 3'
[0068] 3' - - - - - - - - - 5'
[0069] 2 4 6 8 10 12 14 16 18 20
[0070] To complete the synthesis of the c...
Embodiment 2
[0105] Embodiment 2. Utilize goat anti-SARS coronavirus N protein polyclonal antibody to carry out double antibody sandwich analysis
[0106] (1) Preparation of polyclonal antibodies
[0107] According to conventional methods, goats were immunized with the full-length SARS virus N protein prepared in Example 1, and boosted. Polyclonal antibodies were isolated from immunized goat sera and purified with ammonium sulfate and dialyzed against PBS. precipitation.
[0108] (2) Conjugate polyclonal antibodies to colloidal gold particles
[0109] Using the colloidal gold labeling kit of Arista Biologicals Inc., the goat polyclonal antibody against SARS virus N protein was conjugated to 40nm colloidal gold particles for use.
[0110] (3) Prepare test strips in a conventional manner, wherein the goat anti-SARS coronavirus N protein polyclonal antibody conjugated with colloidal gold is the first antibody, and the simple goat anti-SARS virus N protein polyclonal antibody is used as "ca...
Embodiment 3
[0111] The preparation of embodiment 3 mouse anti-SARS-N protein monoclonal antibody:
[0112] I. Antigen preparation: The full-length SARS-N recombinant protein antigen prepared in Example 1 was dissolved in 1×PBS to prepare a 0.5 mg / ml working solution.
[0113] II. Mice: Balb / c female mice, 5-6 weeks old. Immunization begins at 8-9 weeks of age.
[0114] III. Preparation of hybridoma:
[0115] 1). Mix 1.5ml of full-length SARS-N protein with a concentration of 1.0mg / ml and an equal volume of complete Freund's adjuvant.
[0116] 2). Primary immunization of mice: each mouse was injected with 0.5ml, and a total of 6 mice were immunized.
[0117] 3). Booster immunization: start booster immunization on the 14th day after the initial immunization, and then booster immunization every 14 days. The booster immunization was carried out with a mixture of antigen and incomplete Freund's adjuvant, and a total of 3 booster immunizations were performed. On the 10th day after the thir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com