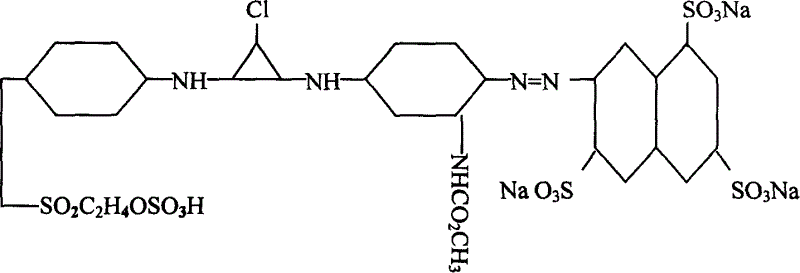
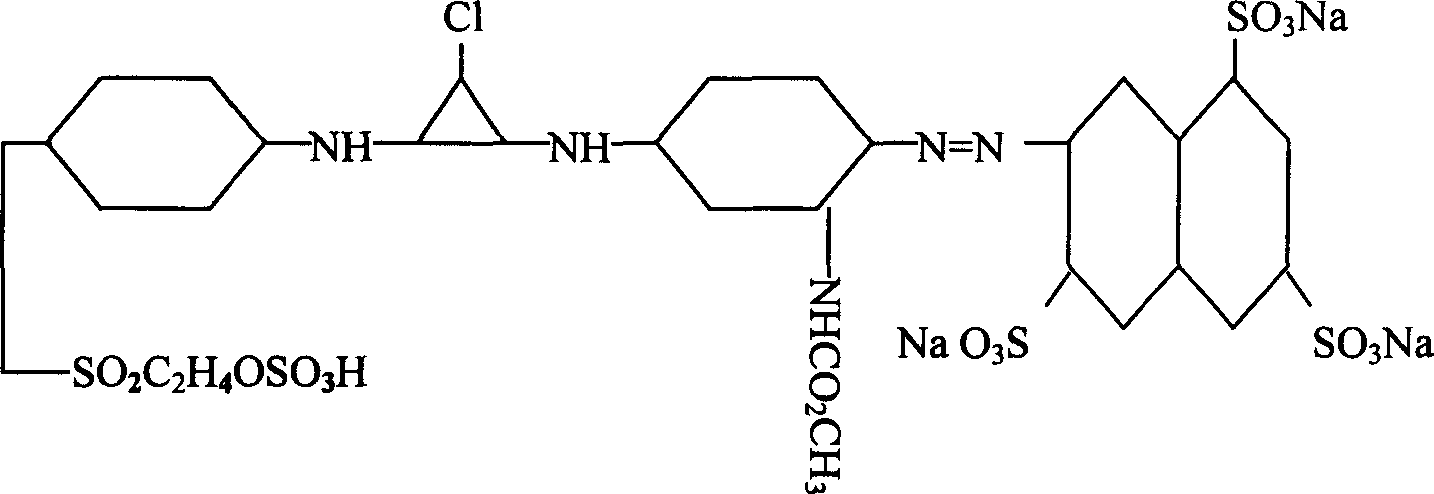
Active yellow SDE, synthetic method and its composite active yellow dye
A synthesis method and technology of reactive yellow, applied in the field of dyes, can solve the problems of increasing production input and cost, dyes cannot be dyed dark colors, poor directness, etc., to reduce the consumption of heat and electric energy, comprehensive product quality, and improve the content of the product. solid effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1, the active yellow monomer adopts 100 parts of 3,6,8-trisulfonic acid-2-naphthylamine dry product to beat evenly in the presence of ice, and then mix it with hydrochloric acid and sodium nitrite at about 5-8°C. After the diazotization reaction, add 105 parts of m-aminoaniline acetylated hydrochloride to the diazo compound at 5-10°C and PH value of 4-6 to carry out the coupling reaction, and then combine with 105 parts of the Cyanuric chloride is condensed once at about 0-5°C, keeping the temperature at 0-15°C, and the pH value is 4-6.5. After the reaction is completed, add 104 parts of dry p-(β-sulfate ethyl sulfone) aniline for a second time Condensation, control temperature 40-65 ℃, pH value 5-7, after the reaction is completed, filter and spray dry directly.
Embodiment 2
[0011] Example 2, the active yellow monomer adopts 102 parts of 3,6,8-trisulfonic acid-2-naphthylamine dry product to beat evenly in the presence of ice, and then mix it with hydrochloric acid and sodium nitrite at about 5-8°C After the diazotization reaction, add 102 parts of m-aminoaniline acetylated hydrochloride to the diazo compound at 5-10°C and PH value of 4-6 to carry out the coupling reaction, and then combine with 103 parts of the Cyanuric chloride is condensed once at about 0-5°C, keeping the temperature at 0-15°C, and the pH value is 4-6.5. After the reaction is completed, add 106 parts of dry p-(β-sulfate ethyl sulfone) aniline for a second time Condensation, control temperature 40-65 ℃, pH value 5-7, after the reaction is completed, filter and spray dry directly.
Embodiment 3
[0012] Embodiment 3, composite reactive yellow dyestuff, is made up of 90 parts of reactive yellow SDE monomer, 2 parts of dustproof agent, 8 parts of sodium sulfate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com