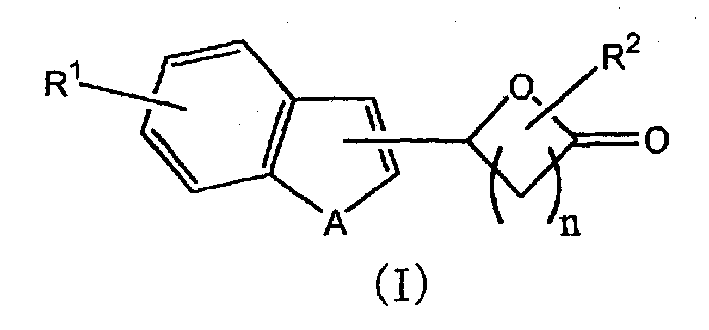
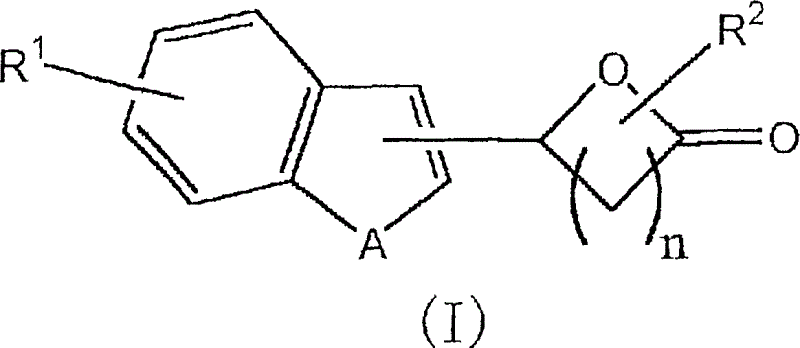
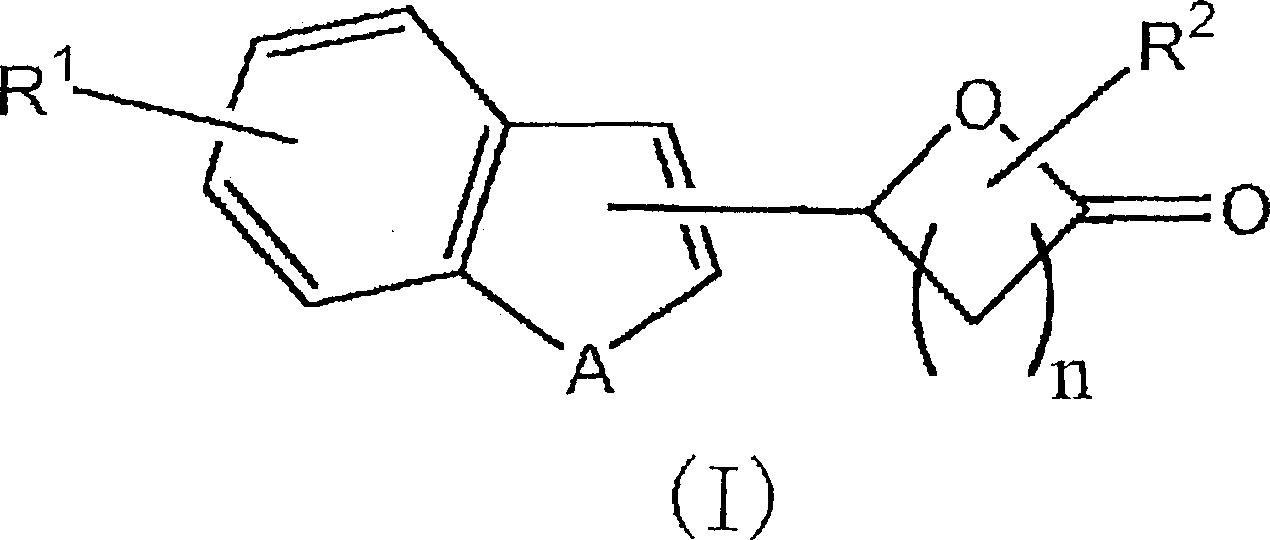
Lactone derivative, and plant growth regulator and rooting agent each containing the same as active ingredient
A technology of derivatives and lactones, applied in the field of lactone derivatives, can solve the problem of weak effect of hair root agent IBA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0043] The present invention will be described in detail below by way of examples.
[0044] Hair root induction activity test
[0045] (a) Plants and cultivation conditions
[0046] Evaluation of hairy root activity was performed using adventitious roots of Bupleurum falcatum L. . The adventitious roots were induced from the roots of Bupleurum bupleuri seedlings by using IBA, and the B5 liquid medium (3 weeks) containing 5 mg / l IBA was alternately cultured at 23°C with the same medium without IBA (6 weeks). The root inoculation amount was 5 g / l, the culture temperature was 23° C., and subculture was carried out under the condition of shaking at 100 rpm in the dark.
[0047] (b) Hair root test using Bupleurum root (1)
[0048] In the hairy root test, adventitious roots cultured in a medium without adding IBA were used. Add 30ml of B5 medium to a 100ml flask, implant 0.2g of Bupleurum root into the medium under sterile conditions, and add the prepared 10,000ppm of the lacto...
preparation example 1
[0060] 5-(1-benzofuran-2-yl)dihydro-2(3H)-furanone (compound 1)
[0061]
[0062] Dissolve 2,3-benzofuran (3.0g, 25.4mmol) in dichloromethane, add ethyl-4-chloro-4-oxobutyrate (4.0ml, 27.9mmol), BF3·Et20 (3.9 ml, 30.5mmol), stirred at 0°C for 10 minutes. Tin tetrachloride (3.6ml, 27.9mmol) was added dropwise, stirred at 0°C for 30 minutes, and then at room temperature for 2 hours. 3N hydrochloric acid was slowly added to the reaction solution under ice cooling, followed by extraction with chloroform. The extract was washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The obtained crude product was adsorbed on silica gel column chromatography (n-hexane:ethyl acetate=5:1) to obtain ethyl 4-(1-benzofuran-2-yl)-4-oxobutyrate (1.88g , 30%). Dissolve ethyl 4-(1-benzofuran-2-yl)-4-oxobutyrate (1.85g, 7.51mmol) in dioxane-water (1:1), add 1M Lithium hydroxide (15ml, 15mmol). After stirring at room temperature for 1 hour, ...
preparation example 2
[0066] 5-(1-benzothiophen-3-yl)dihydro-2(3H)-furanone (compound 2)
[0067]
[0068] Benzo[b]thiophene (2.00g, 14.9mmol) was dissolved in dichloromethane, ethyl 4-chloro-4-oxobutyrate (2.34ml, 16.4mmol) was added, and stirred at 0°C for 10 minutes. Tin tetrachloride (2.09ml, 17.9mmol) was added dropwise, stirred at 0°C for 30 minutes, and then at room temperature for 2 hours. Under ice-cooling, 3N hydrochloric acid was slowly added to the reaction liquid, and extracted with chloroform. The extract was washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The obtained crude product was adsorbed on silica gel column chromatography (n-hexane:ethyl acetate=5:1) to obtain ethyl 4-(1-benzothiophen-3-yl)-4-oxobutanoate (3.13g, 80%).
[0069] Dissolve ethyl 4-(1-benzothiophen-3-yl)-4-oxobutyrate (3.10 g, 11.8 mmol) in dioxane-water (1:1), add 1M Lithium hydroxide (23.6ml, 23.6mmol), stirred at room temperature for 30 minutes....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com