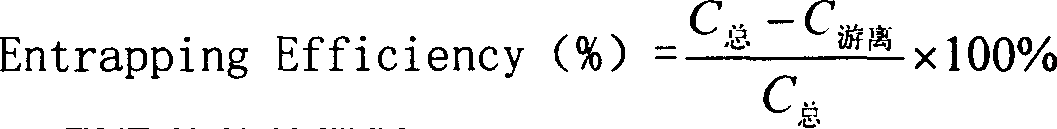Notoginsen triterpenes liposome and prepartion thereof
The technology of total saponins and total saponins of Panax notoginseng is applied in the field of medicine, which can solve the problems of increased side effects, low curative effect, poor permeability and the like, and achieve the effects of improving poor oral absorption, enhancing curative effect and improving compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of Panax notoginseng liposomes by thin film method
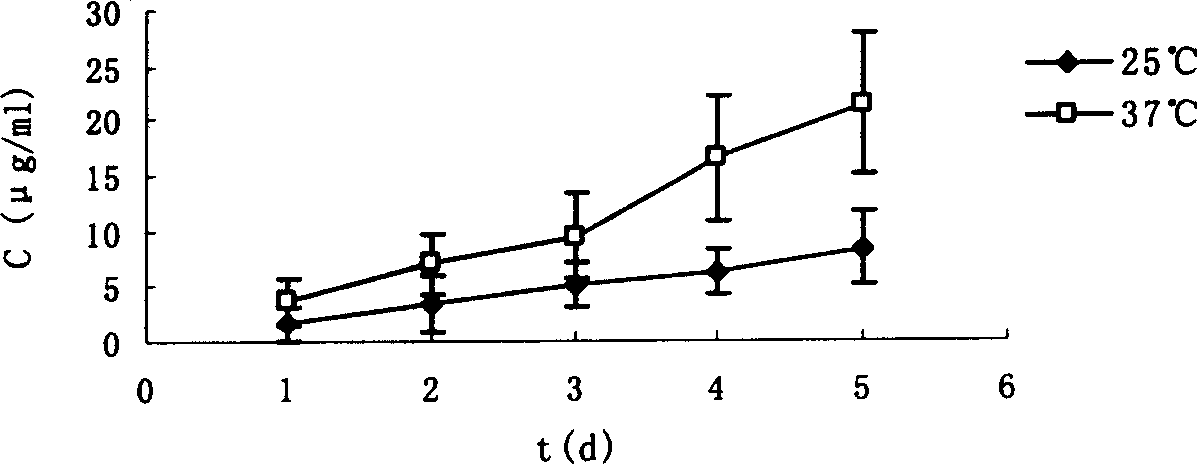
[0022] Get 1.25 grams of soybean lecithin (Shanghai Taiwei Pharmaceutical Co., Ltd.), 0.3 grams of cholesterol (Shanghai Chemical Reagent Company, China Pharmaceutical Group) and dissolve it in a 250 ml pear-shaped bottle with 60 milliliters of ether, and evaporate the ether under reduced pressure on a rotary evaporator , the lipid forms a thin film on the wall of the bottle, add 15ml of Panax notoginsenosides phosphate buffer and spin hydration to obtain the Panax notoginseng saponins liposomes. The encapsulation efficiency was 81%.
Embodiment 2
[0023] Example 2 Preparation of Panax notoginseng saponins liposomes by reverse phase evaporation
[0024] Dissolve 2 grams of soybean lecithin and 1 gram of cholesterol in a 250 ml pear-shaped bottle with 100 ml of ether, then add 20 ml of Panax notoginseng phosphate buffer, ultrasonically in a water bath to form a homogeneous single-phase system, and evaporate under reduced pressure to remove the ether until it solidifies. After the gel was formed, continue to evaporate under reduced pressure for 15 minutes to obtain liposomes of Panax notoginseng saponins. The encapsulation efficiency is 60%.
Embodiment 3
[0025] Example 3 Preparation of Notoginseng Total Saponins Liposomes by Freeze-drying
[0026] Dissolve 1.25 grams of soybean lecithin and 0.3 grams of cholesterol in a 250 ml pear-shaped bottle with 60 milliliters of ether, evaporate the ether under reduced pressure on a rotary evaporator, the lipid forms a film on the wall of the bottle, add 15 ml of phosphate buffer saline and spin water To obtain a blank liposome, add an appropriate amount of trehalose, mix well, freeze-dry in a vacuum, and then disperse by shaking with Panax notoginseng phosphate buffer solution to obtain Panax notoginseng liposome. The encapsulation efficiency was 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com