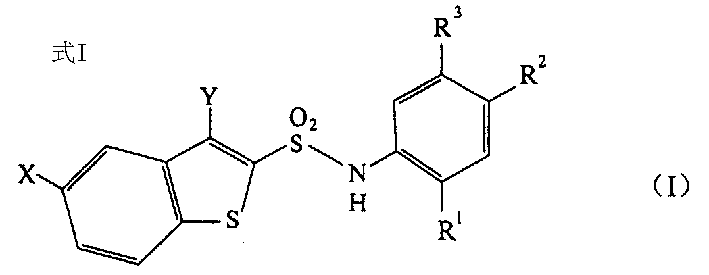
N-substituted benzothiophenesulfonamide derivatives
A technology of benzothiazole and derivatives, which is applied in the field of chymotrypsin inhibitors, can solve the problem of no chymotrypsin inhibitors being found, and achieve the effect of strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Methyl 4-(5-chloro-3-methylbenzo[b]thiophen-2-sulfonylamino)-3-methanesulfonylbenzoate
[0064] In a mixed solvent of 20 mL of THF and 3 mL of DMF, 985 mg of methyl 4-amino-3-methanesulfonyl benzoate was dissolved, and then 170 mg of sodium hydride (oily, 60%) was added at 0°C. After stirring at this temperature for 20 minutes, 1.28 g of 5-chloro-2-chlorosulfonyl-3-methylbenzo[b]thiophene was added at 0°C, followed by stirring at room temperature for 1 hour. Furthermore, 150 mg of sodium hydride (oily, 60%) was added at room temperature, and then the mixture was stirred at this temperature for 2 hours. After confirming the disappearance of the reactant, saturated ammonium chloride aqueous solution was added at 0°C to terminate the reaction, and then extraction was performed with ethyl acetate. The organic layer was washed with saturated brine, and then dried over anhydrous sodium sulfate. The solvent was removed by evaporation, and the residue was purified by silica gel chr...
Embodiment 2
[0069] Ethyl 4-(5-chloro-3-methylbenzo[b]thiophene-2-sulfonylamino)-3-methanesulfonylbenzoate
[0070] In the same manner as in Example 1, 559 mg of ethyl 4-amino-3-methanesulfonylbenzoate was used to obtain 529 mg of the title compound as a colorless powder.
[0071] Melting point: 167-169°C
[0072] 1 H-NMR(CDCl 3 ): δ 1.36 (3H, t, J = 7.1 Hz), 2.70 (3H, s), 3.06 (3H, s), 4.36 (2H, q, J = 7.1 Hz), 7.47 (1H, dd, J = 2.0, 8.8 Hz), 7.74 (1H, d, J = 8.8 Hz), 7.78 (1H, d, J = 2.0 Hz), 7.86 (1H, d, J = 8.8 Hz), 8.19 (1H, dd, J = 2.0, 8.8 Hz), 8.50 (1H, d, J=2.0 Hz), 9.83 (1H, brs).
[0073] IRν max (KBr): 3224, 2985, 1716, 1608, 1500, 1358, 1300, 1142cm -1 .
Embodiment 3
[0075] Tert-Butyl 4-(5-chloro-3-methylbenzo[b]thiophen-2-sulfonamido)-3-methanesulfonylbenzoate
[0076] According to the same method as in Example 1, 128 mg of tert-butyl 4-amino-3-methanesulfonylbenzoate was used to obtain 148 mg of the title compound as a colorless powder.
[0077] Melting point: 236-238°C
[0078] 1 H-NMR(CDCl 3 ): δ 1.54 (9H, s), 2.52 (3H, s), 3.28 (3H, s), 7.55-7.80 (4H, m), 8.00 (1H, s), 8.25-8.30 (1H, m).
[0079] IRν max (KBr): 3467, 2974, 2327, 1705, 1662, 1597, 1477, 1396, 1296, 1130, 1099cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com