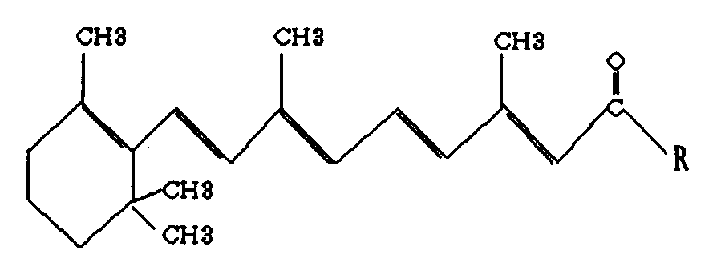
Compound N-total trans dimension methanamide derivative and its preparation method and application
A retinoic acid, all-trans technology, applied in the chemical field, can solve the problems of retinoic acid not reaching the effective inhibitory concentration, liver function damage, easy recurrence, etc., to achieve inhibition of metastasis potential, high degree of anti-malignancy, and low survival rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
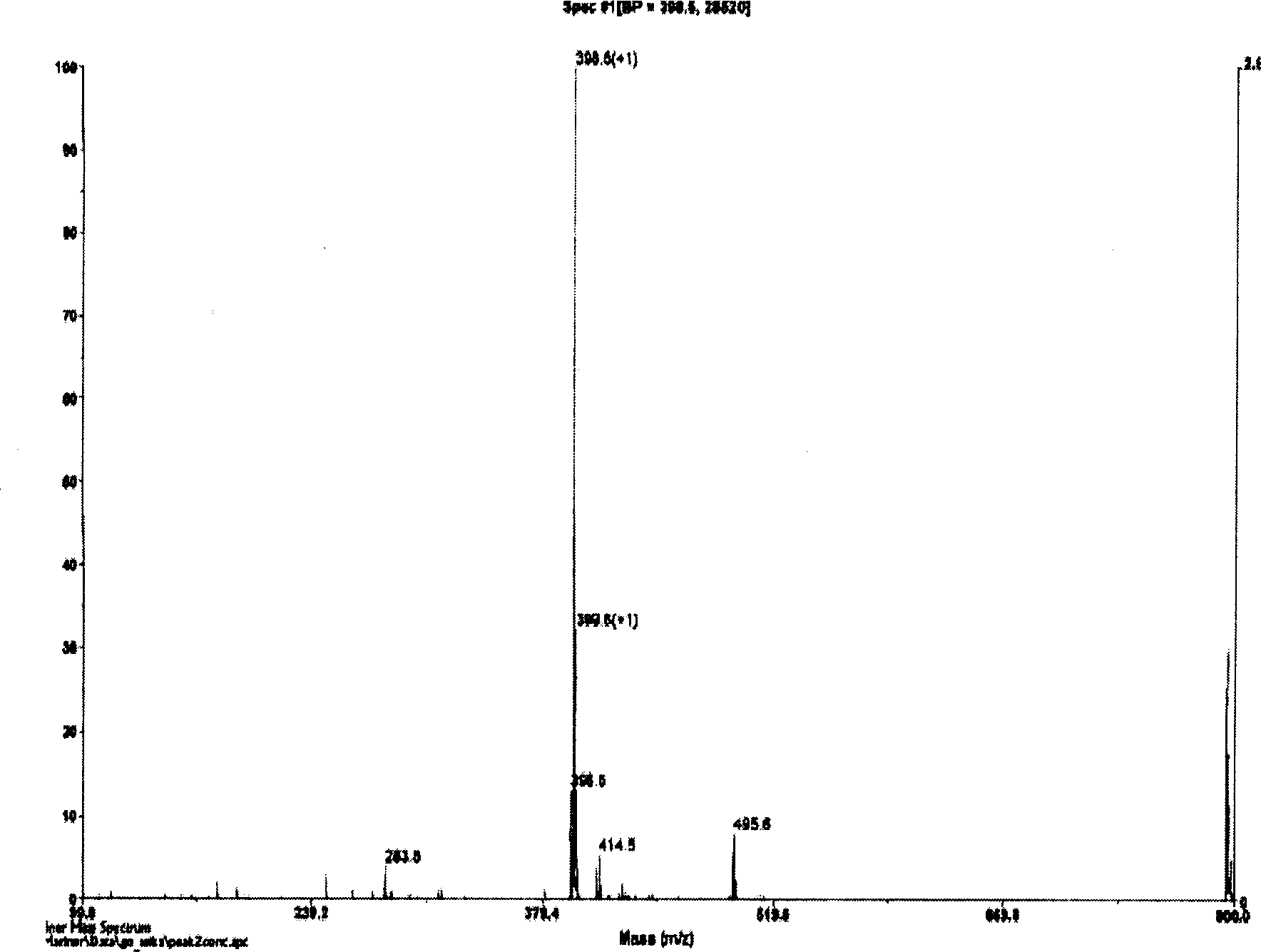
Image
Examples
Embodiment 1
[0032] Use all-trans retinoic acid dry powder as a synthetic raw material, react with phenol and phosphorus trichloride, and stir for 2.5 hours under the protection of nitrogen, an orange-red solution appears, and a small amount of phosphorous acid precipitates on the wall of the reaction bottle. Stir under protection for 15 minutes, transfer to a dry layered funnel, separate the benzene solution that generates retinoyl chloride, stir in an ice bath for 10 minutes, add dropwise ice-cold L-proline, which has been pre-prepared Dissolve in 2mol / l sodium hydroxide solution, continue to stir, and adjust the pH of the solution with 2mol / l sodium hydroxide solution, keep it above 9.0, remove the ice bath, stir the reaction solution overnight, and divide the reaction solution into Two layers, the upper layer is brownish-yellow, the lower layer is darker in color, extracted three times with ether, the upper layer is added to a silica gel column for chromatography, samples are collected ...
Embodiment 2
[0034] Functional test of the compound of the present invention
[0035] 1. Effects on the cycle of liver cancer cells:
[0036] Hep3B liver cancer cells were treated with different concentrations, and then the cell cycle was detected by flow cytometry to observe the effect of the compound N-all-trans retinamide proline (ATRP) on the cycle of liver cancer cell Hep3B cells. The results showed that N-all Trans-retinoic acid amide proline has an effect at the beginning of 1 μmol / l, and there is a significant difference in the change of cell cycle when it is 3 μmol / l. The period of action is mainly in the G2-M phase, which increases the ratio of cells in the G2-M phase. Even liver cancer cells stay in the G2-M phase more often. Table 1 is the cell cycle analysis.
[0037] 2. Effect on the expression of Lewis antigen on the surface of liver cancer cells:
[0038]N-all-trans retinamide proline acts on Hep3B liver cancer cells for 1-3 days, and then detects and observes the expres...
Embodiment 3
[0046] The compound N-all-trans retinamide proline of the present invention was compared with all-trans retinoic acid (ATRA) and 4-aminophenol retinamide (4HPR). All-trans retinoic acid 10μM concentration acts on Hep3B cells for 3 days, 4-aminophenol retinamide and N-all-trans retinoic acid amide proline are both 3 μM on Hep3B cells for 3 days, and the cells are collected for enzyme activity. The results showed that after Hep3B liver cancer cells were treated with N-all-trans-retinamide proline for 3 days, the α-1,6FT activity increased from 150.51±68.94 in the control to 266.47±11.91pmol / hr·mg, the two groups The difference was significant (P=0.0007). However, there is no significant difference in the effects of all-trans retinoic acid and 4-aminophenol retinoic acid, indicating that the compound of the present invention has a strong regulatory effect on liver cancer cell α-1, 6FT activity, and the concentration of action is 3.3 times lower than that of ATRA , while all-tran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com