Compound N-total trans dimension methanamide derivative and its preparation method and application
A retinoic acid, all-trans technology, applied in the field of chemistry, can solve the problems of retinoic acid not reaching the effective inhibitory concentration, liver function damage, toxic and side effects, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
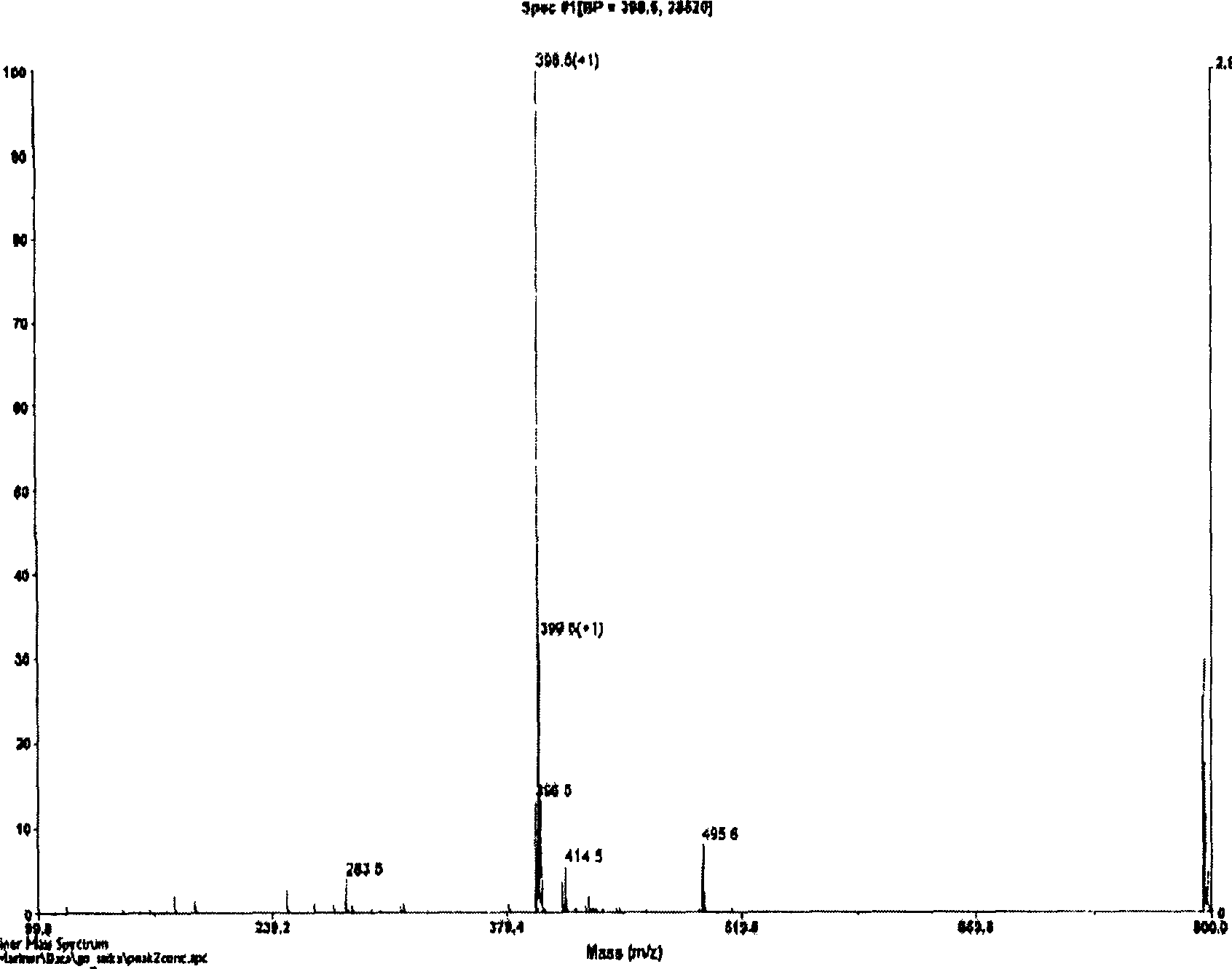
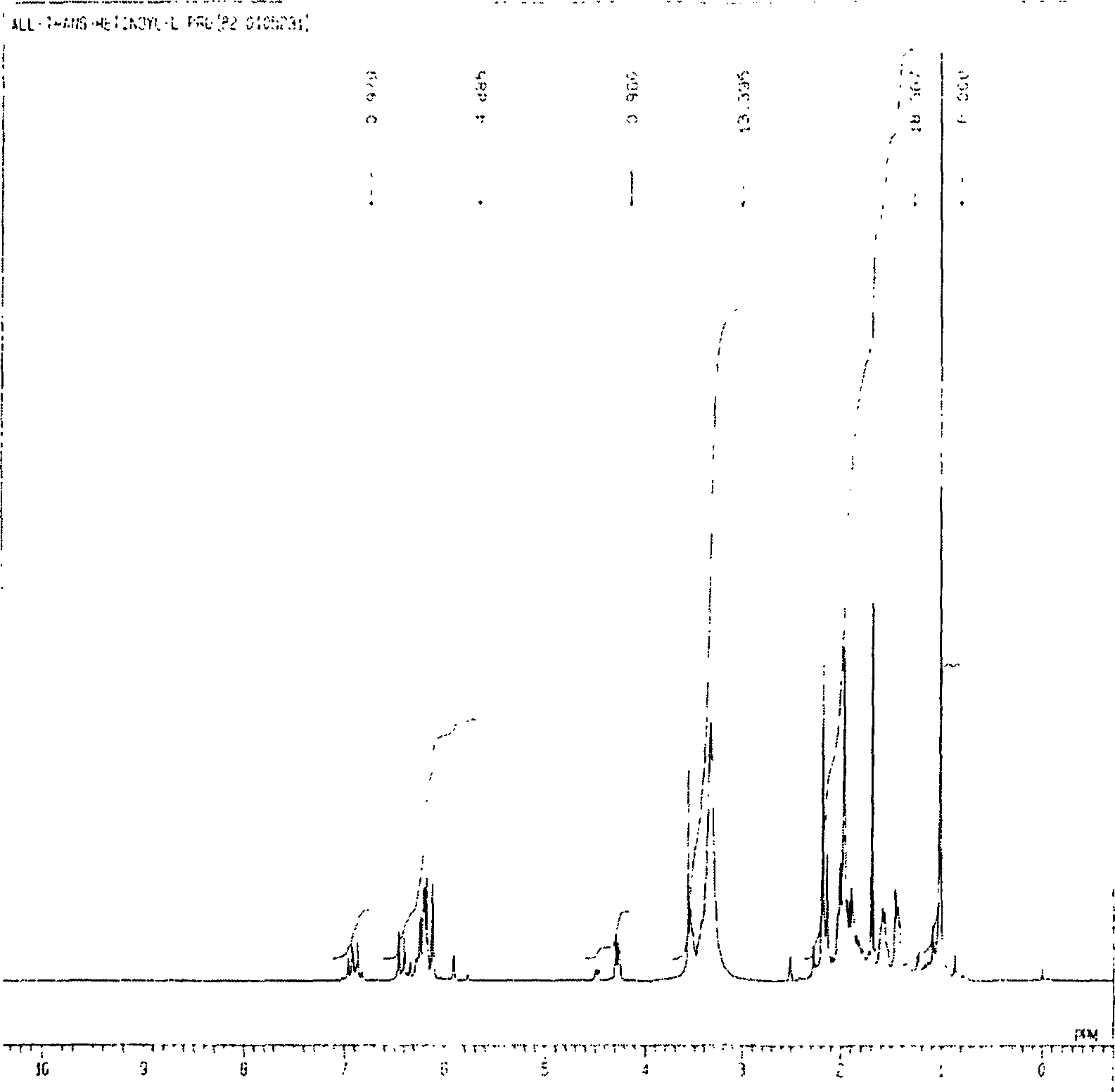
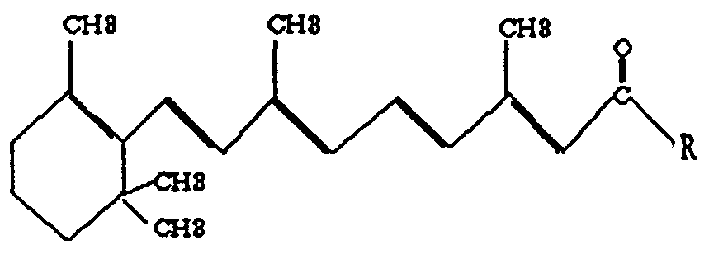
Image
Examples
Embodiment 1
[0033] Use all-trans retinoic acid dry powder as synthetic raw material, react with benzene and phosphorus trichloride, and stir for 2.5 hours under the protection of nitrogen, an orange-red solution appears, a small amount of phosphorous acid precipitates on the wall of the reaction bottle, and continues to Stir under protection for 15 minutes, transfer to a dry layered funnel, separate the benzene solution that generates retinoyl chloride, stir in an ice bath for 10 minutes, add dropwise ice-cold L-proline, which has been pre-prepared Dissolve in 2m01 / l sodium hydroxide solution, continue to stir, and adjust the pH of the solution with 2m01 / l sodium hydroxide solution, keep it above 9.0, go to the ice bath, stir the reaction solution overnight, and divide the reaction solution into Two layers, the upper layer is brownish-yellow, the lower layer is darker in color, extracted three times with ether, the upper layer is added to a silica gel column for chromatography, samples are...
Embodiment 2
[0035] Functional test of the compound of the present invention
[0036] 1. Effects on the cycle of liver cancer cells:
[0037] Hep3B liver cancer cells were treated with different concentrations, and then the cell cycle was detected by flow cytometry to observe the effect of N-all-trans retinamide proline (ATRP) on the cycle of liver cancer cell Hep3B cells. The results showed that N-all-trans Retinamide proline has an effect at 1 μmol / l, and there is a significant difference in the change of cell cycle when it is 3 μmol / l. The period of action is mainly in the G2-M phase, which increases the ratio of G2-M phase cells. Even liver cancer Most of the cells stay in the G2-M phase. Table 1 is the cell cycle analysis.
[0038] 2. Effect on the expression of Lew" antigen on the surface of liver cancer cells:
[0039] N-all-trans retinamide proline acts on Hep3B liver cancer cells for 1-3 days, and then detects and observes the expression of Lewis antigen on the cell surface with ...
Embodiment 3
[0047] N-all-trans retinamide proline of the present invention was compared with all-trans retinoic acid (ATRA) and -4-aminophenol retinamide (4HPR). All-trans retinoic acid 10μM concentration acts on Hep3B cells for 3 days, 4-aminophenol retinamide and N-all-trans retinoic acid amide proline are both 3 μM on Hep3B cells for 3 days, and the cells are collected for enzyme activity. As a result, after Hep3B liver cancer cells were treated with N-all-trans-retinamide proline for 3 days, the activity of α-1,6FT increased from 150.51±68.94 in the control to 266.47±11.91pmol / hr.mg, the two groups The difference was significant (P=0.0007). However, there is no significant difference in the effects of all-trans retinoic acid and 4-aminophenol retinoic acid, indicating that the compound of the present invention has a strong regulatory effect on liver cancer cell α-1, 6FT activity, and the concentration of action is 3.3 times lower than that of ATRA , while all-trans retinoic acid and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com