Method for measuring coagulant factor activity in whole blood
A technology of blood and factors, applied in the field of medical diagnostics and disease prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
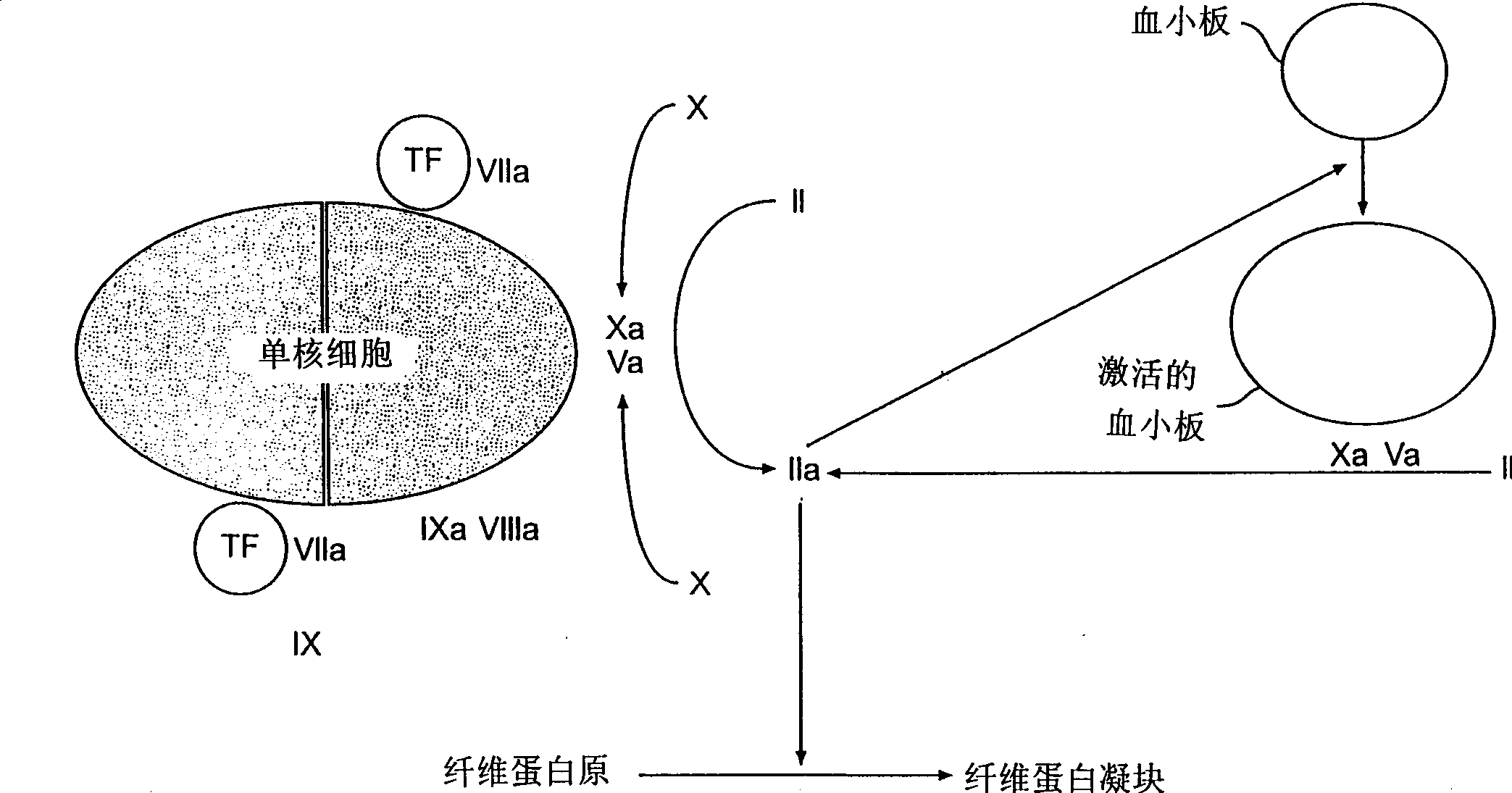
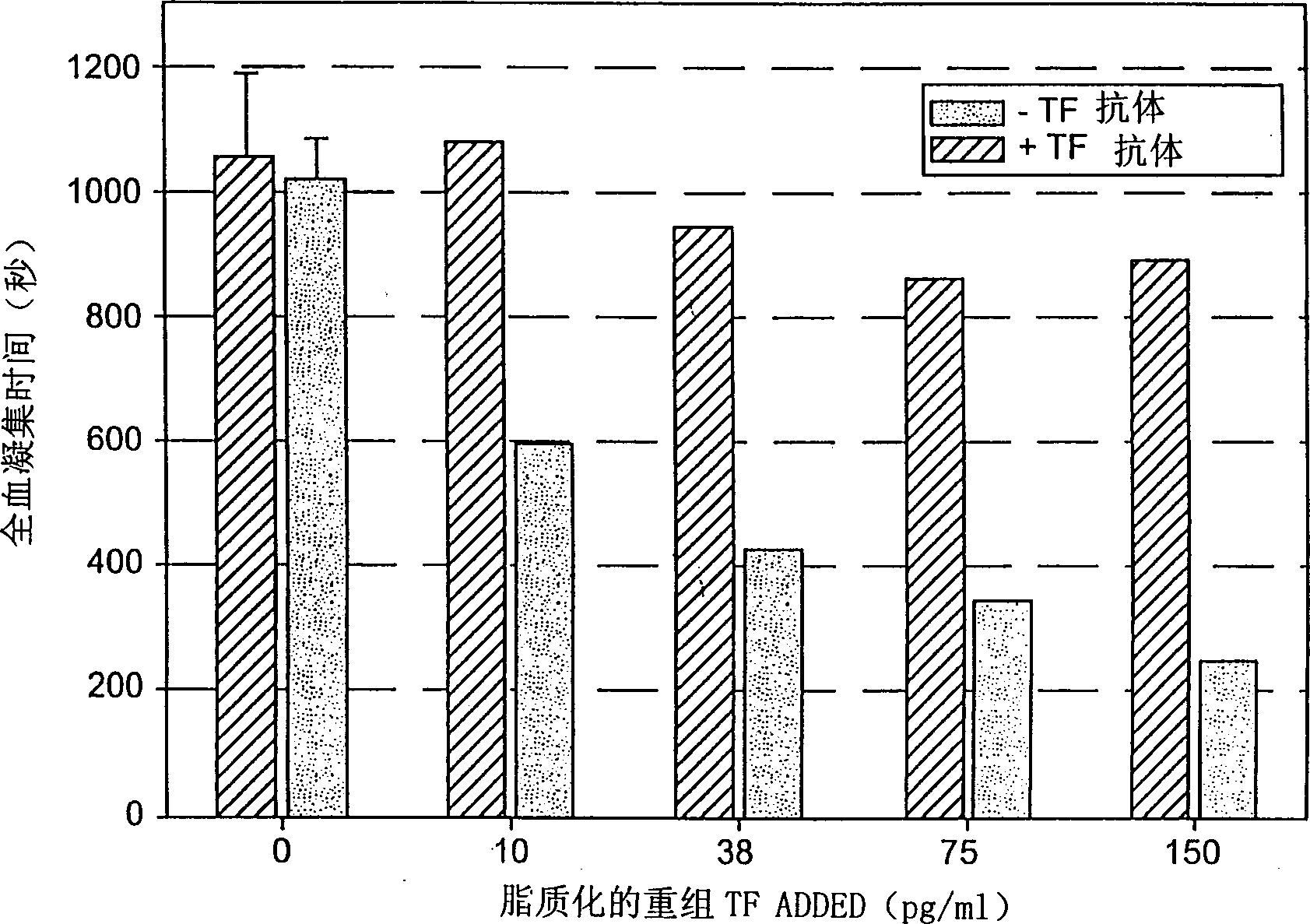
[0059] The whole blood assay for tissue factor, described in this example, involved a protocol performed on a Hemochron instrument. This procedure used an anti-TF antibody inhibition assay to assess endogenous circulating tissue factor levels in whole blood. Materials and Reagents Required for Evaluation of TF in Circulation
[0060] Hemochron P213 Sample Tubes
[0061] 0.01M calcium chloride stock solution
[0062] Control vials containing non-inhibiting antibodies
[0063] Vial containing dried anti-tissue factor antibody
[0064] Test Quality Control Reagents
[0065] Hemoliance RecombiPlasTin stock solution (lipidated recombinant tissue factor)
[0066] TF Diluent (20mM HEPES 150mM NaCl, pH7.4, containing 0.10mg / mL
[0067] bovine serum albumin)
[0068] Blood containing anticoagulant citrate fluid and corn trypsin inhibitor (CTI)
[0069] Collection Tube Hemochron P213 Tube Preparation
[0070] Hemochron P213 tubes were preloaded with 50 μL of 0.10 M calcium chlo...
Embodiment 2
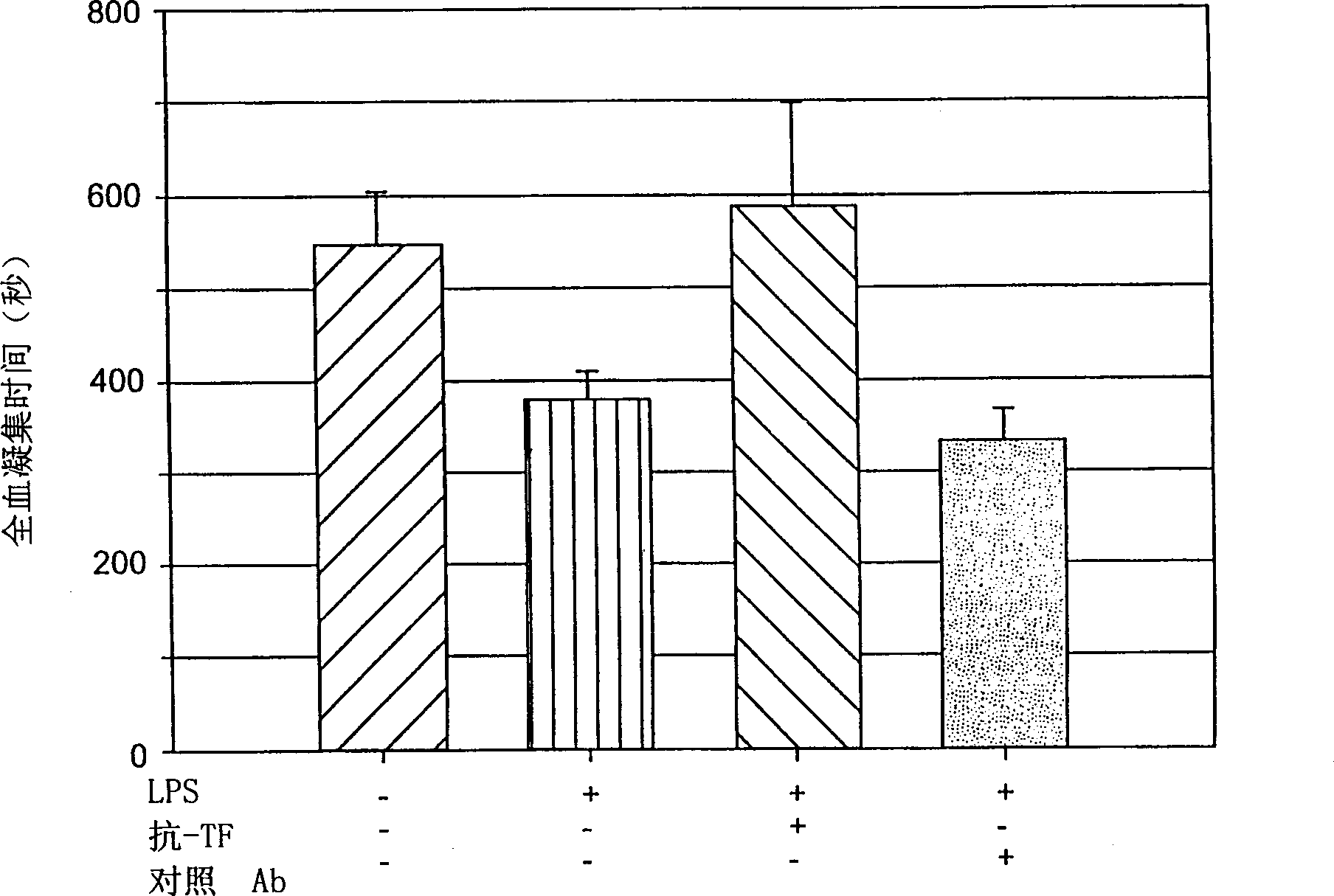
[0094] This example describes the procedure for a lipopolysaccharide-stimulation assay performed on a Hemochron instrument that evaluates the production of reactive TF in whole blood following endotoxin (lipopolysaccharide) stimulation. Materials and reagents required to evaluate reactively produced TF in whole blood following LPS stimulation
[0095] Hemochron P213 Sample Tubes
[0096] 0.10M calcium chloride stock solution
[0097] Vials containing dried LPS
[0098] Vials containing dried LPS and dried anti-tissue factor antibody
[0099] Blood containing anticoagulant citrate fluid and corn trypsin inhibitor (CTI)
[0100] Collection Tube Hemochron P213 Tube Preparation
[0101] Hemochron P213 tubes were preloaded with 50 μl of 0.10 M calcium chloride solution before the stimulated tissue factor clotting time test. Store the tubes capped at room temperature. draw blood
[0102] Discard the first few milliliters of blood drawn, then draw blood into the 5ml citrate / CTI ...
Embodiment 3
[0112] The tissue factor whole blood assay described in this example consists of a Sonoclot TM Experimental operations performed on the instrument. This procedure used an anti-TF antibody inhibition assay to assess tissue factor levels in LPS-stimulated whole blood. sample requirements
[0113] Using a 19 mm graduated needle, follow the phlebotomy procedure as detailed in, e.g., Collection, Transport, and Processing of Blood Specimens for Coagulability Testing and Coagulability Assay Procedures (National Committee on Clinical Laboratory Standards document, # H21-A-2, Volume 11, Issue 23) ((Collection.Transport and Processing of Blood Specimens for Coagulation Testing and Performance of Coagulation Assays) National Committee for Clinical Laboratory Standards document#H21-A-2, Volume XI, No. twenty three). First collect a discard tube (the top is a blue Vacutainer), and then put a plastic Vacutainer tube containing 50 μg / ml corn trypsin inhibitor (CTI) and 0.5ml 3.2% sodium c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com