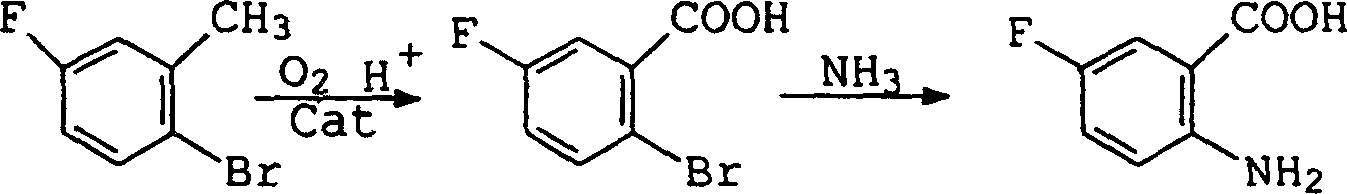
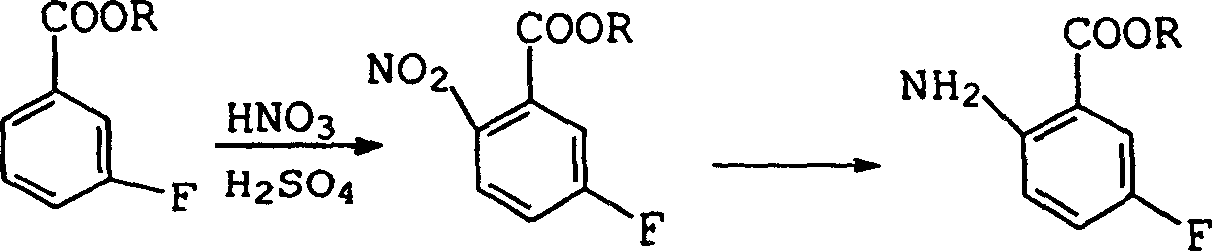
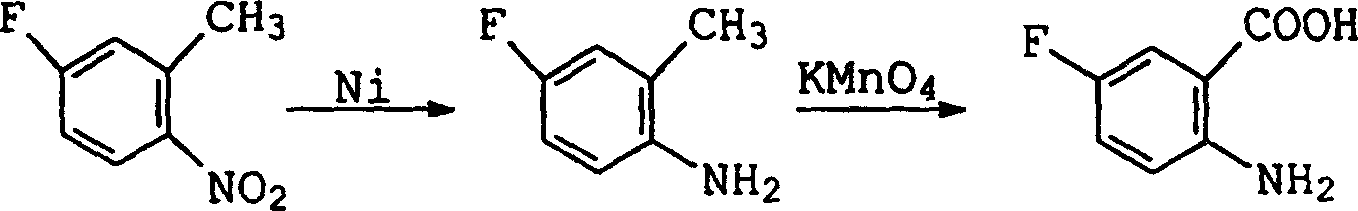
Preparation method of 2-amino-5-fluorobenzoic acid
The technology of fluorobenzoic acid and amino group is applied in the field of preparation of 2-amino-5-fluorobenzoic acid, and can solve the problems of difficulty in obtaining, decrease in alkalinity, decrease in reaction yield, etc., and achieves low cost and easy availability of raw materials and reaction conditions. Gentle, simple and safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First prepare the chloral hydrate solution, weigh 18.2 g of chloral hydrate, and dissolve it in 400 mL of water. Then prepare 4-fluoroaniline solution, weigh 11.1 g of 4-fluoroaniline and 22.3 g of hydroxylamine hydrochloride, pour them into 160 mL of water, add 9 mL of concentrated hydrochloric acid, and stir until a yellow and clear transparent solution is obtained.
[0025] Add the prepared chloral hydrate solution to the 1000mL three-necked flask equipped with mechanical stirring, spherical condenser and thermometer, and heat up the oil bath. Add 260 grams of anhydrous sodium sulfate during the heating process. The solution was milky white, and sodium sulfate could not be completely dissolved. After adding the prepared 4-fluoroaniline solution, white flocculent precipitates appeared immediately, and the temperature was raised to reflux. After reacting for 2 hours, the mixture was filtered under reduced pressure while hot to obtain a brown-yellow filter cake. The f...
Embodiment 2
[0027] Same as Example 1, after the feeding is completed, the temperature is raised to 95° C., reacted for 2 hours, and the aftertreatment is the same as above. A white solid (12.6 g of N-(4-fluorophenyl)-2-(hydroxyimine)acetamide) was obtained, yield: 69.3%.
Embodiment 3
[0029] Same as Example 1, after the feeding is completed, the temperature is raised to 95° C., reacted for 3 hours, and the aftertreatment is the same as above. 12.2 g of white solid (N-(4-fluorophenyl)-2-(hydroxyimine)acetamide) was obtained, yield: 67.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com