Development suppressing group-releasing magenta color coupler
A coupler and group technology, applied in the field of silver halide color photosensitive negative materials, can solve the problems of slow release rate of development inhibitor and high synthesis cost, improve image granularity and color reproducibility, low synthesis cost, coupling Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
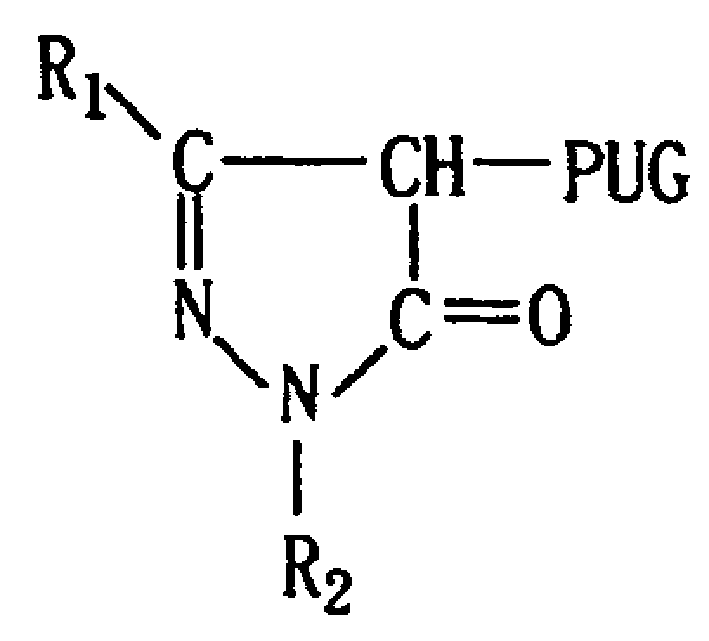
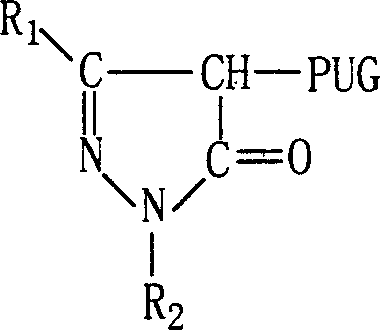
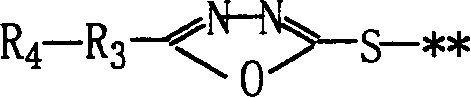
[0029] (Illustrative Compound MCP-ZB): 1-(2,4,6-Trichlorophenyl)-3-[3-[(2,4-Dipentylphenoxy)acetamido]benzamido ]-4-[[5-[(4-methylphenoxy)methyl]-1,3,4-oxadiazol-2-yl]mercapto]-5-pyrazolone.
[0030] 1. Intermediates
[0031] 1-(2,4,6-Trichlorophenyl)-3-[3-(2,4-Di-t-pentylphenoxy)acetamido]benzamido-4-bromo-5-pyrazolone preparation.
[0032] Reaction formula:
[0033]
[0034] (referred to as MCP parent compound)
[0035]
[0036] (referred to as MCP parent bromide)
[0037] Operation method:
[0038] In a 250 ml three-neck flask equipped with a stirrer and a thermometer, add 20 g of the MCP parent compound and 60 ml of DMF (dimethylformamide), cool the temperature of the reaction bottle to 0-5°C, add 1.44 ml of bromine dropwise, and then add 1.44 ml of bromine dropwise for half an hour After the internal dropwise addition is completed, the temperature is naturally raised to room temperature, and the reaction is continued fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com