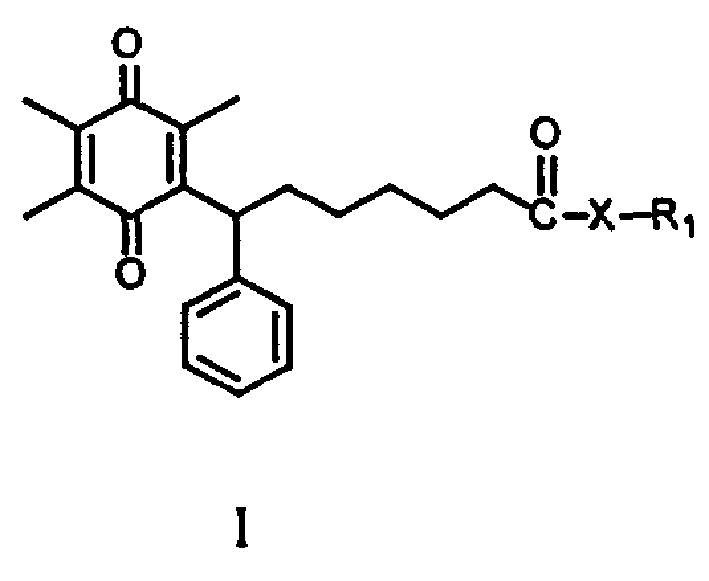
'Sqiqu site' derivative and its preparing method as well as its activity of anti asthma
A technology of sestrolast and derivatives, applied in the fields of sestrolast derivatives and their preparation and anti-asthma activity, can solve problems such as unsatisfactory therapeutic effect of inhibitors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] (±)-N-[3-(2,4-dimethylphenyl)-1,2,3,4-oxatriazol-5-yl]-7-(2,4,5-trimethyl -3,6-dioxo-1,4-cyclohexadien-1-yl)phenylheptimide (I-1A 1 )
[0120] 3-(2,4-Dimethylphenyl)-1,2,3,4-oxatriazole-5-imine hydrochloride (38mg, 0.168mmol), 2mlNaHCO 3 The saturated solution and 2ml of dichloromethane were added to a 25ml two-neck flask, and under nitrogen protection with vigorous stirring, a solution of SD-COCl (about 0.168mmol) dissolved in 2ml of anhydrous dichloromethane was added dropwise. After reacting for 2 hours, separate the organic layer, wash with water (10ml×3), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain a yellow solid, add 5ml of ether and stir for 10 minutes, then suction filter, the filter cake is light yellow powder The solid was dried to obtain 70 mg, yield 79.5%, mp 114°C-116°C.
[0121] IR (KBr, cm -1 ): 1702 (amide), 1642 (C=O);
[0122] ESI-MS: 527[M+H] + , 549[M+Na] + ;
[0123] 1 HNMR (500MHz, CDCl 3 ), δ(ppm): 1...
Embodiment 2
[0125] (±)-7-(2,4,5-trimethyl-3,6-dioxo-1,4-cyclohexadien-1-yl)phenylheptanoic acid (N-4-methylphenyl -N'-hydroxyguanidine) ester (I-2A 1 )
[0126] Add SD (0.1g, 0.28mmol), p-methylphenyl-N-hydroxyguanidine (0.05g, 0.31mmol), DMAP pellets and 5ml of dichloromethane into a 10ml eggplant-shaped bottle, stir to dissolve, then add in batches DCC (0.069g, 0.34mmol) was stirred overnight at room temperature, and a white precipitate formed. Filtration, column chromatography [petroleum ether (60 ° C ~ 90 ° C): acetone: glacial acetic acid = 4: 1: 0.01 (v: v: v)], to obtain a light yellow powdery solid 0.085 g, yield 60.5%, mp 106°C~108°C.
[0127] IR (KBr, cm -1 ): 3376 (NH), 1731 (ester), 1642 (C=O);
[0128] ESI-MS: 502[M+H] + , 524[M+Na] +
[0129] 1 HNMR (300MHz, d-DMSO), δ (ppm): 1.24~1.68 (m, 6H, 3×CH 2 ), 1.87 (s, 3H, CH 3 ), 1.91 (s, 3H, CH 3 ), 1.98 (s, 3H, CH 3 ), 2.10~2.18 (m, 2H, C 6 -CH 2 ), 2.19 (s, 3H, CH 3 ), 2.30~2.35(t, 2H, J=7.5Hz, C 2 -CH 2 ), 4....
Embodiment 3
[0133] (±)-7-(2,4,5-trimethyl-3,6-dioxo-1,4-cyclohexadien-1-yl)phenylheptanoic acid (N-4-chlorophenyl- N'-Hydroxyguanidine) ester (I-2A 2 )
[0134] Refer to I-2A 1 The preparation method is obtained from p-chlorophenyl-N-hydroxyguanidine. Column chromatography [petroleum ether (60°C-90°C): acetone: glacial acetic acid = 4:1:0.01 (v:v:v)] gave a light yellow powdery solid with a yield of 56.8%, mp 44°C-46 ℃.
[0135] IR (KBr, cm -1 ): 3310 (NH), 1731 (ester), 1643 (C=O);
[0136] ESI-MS: 522[M+H] + , 544[M+Na] + ;
[0137] 1 HNMR (300MHz, CD 3 COCD 3 ), δ(ppm): 1.24~1.68(m, 6H, 3×CH 2 ), 2.00 (s, 3H, CH 3 ), 2.02(s, 3H, CH 3 ), 2.03(s, 3H, CH 3 ), 2.10~2.18 (m, 2H, C 6 -CH 2 ), 2.34~2.39(t, 2H, J=7.5Hz, C 2 -CH 2 ), 4.34~4.35(q, 1H, CH), 5.48(s, 2H, NH 2 ), 7.14~7.25(m, 5H, ArH); 7.30~7.32(m, 2H, ArH), 7.49~7.52(m, 2H, ArH);
[0138] Anal.C 29 h 32 ClN 3 o 4 0.4H 2 O Found (%): N 7.46 C 66.12 H 6.67
[0139] Calcd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com