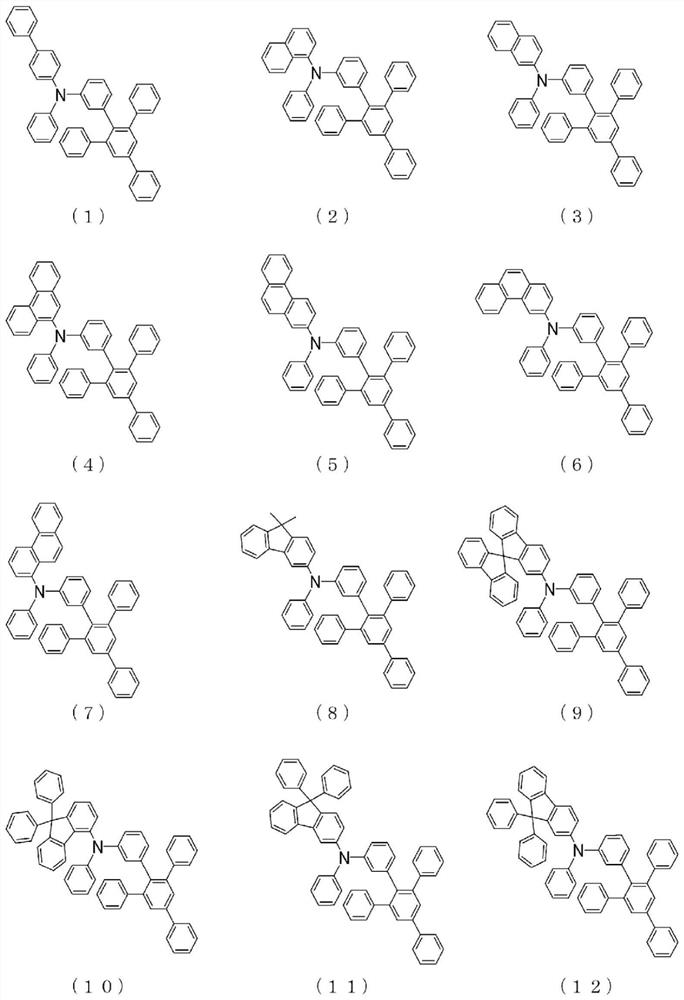
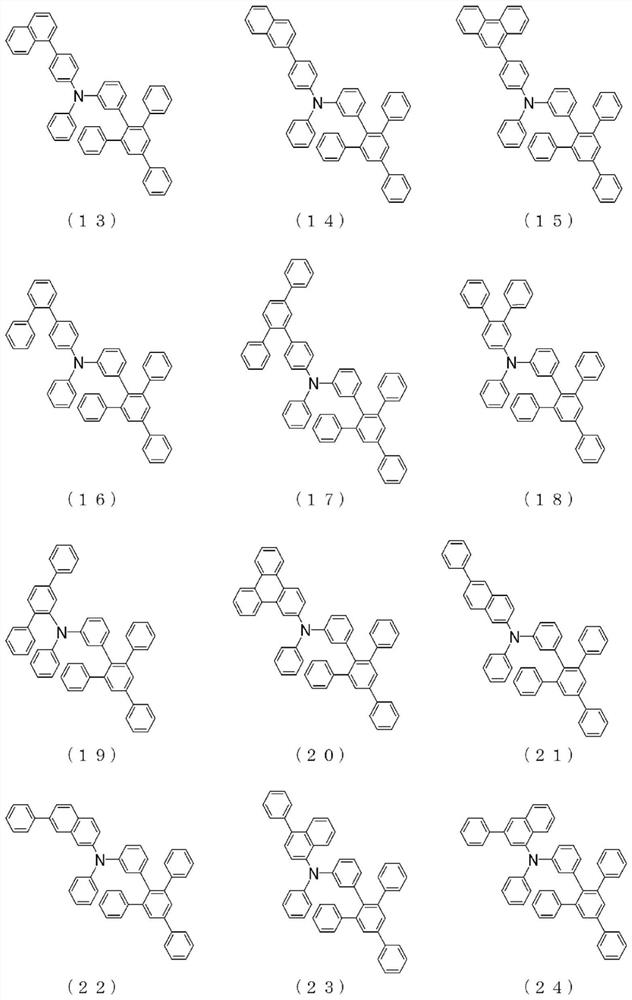
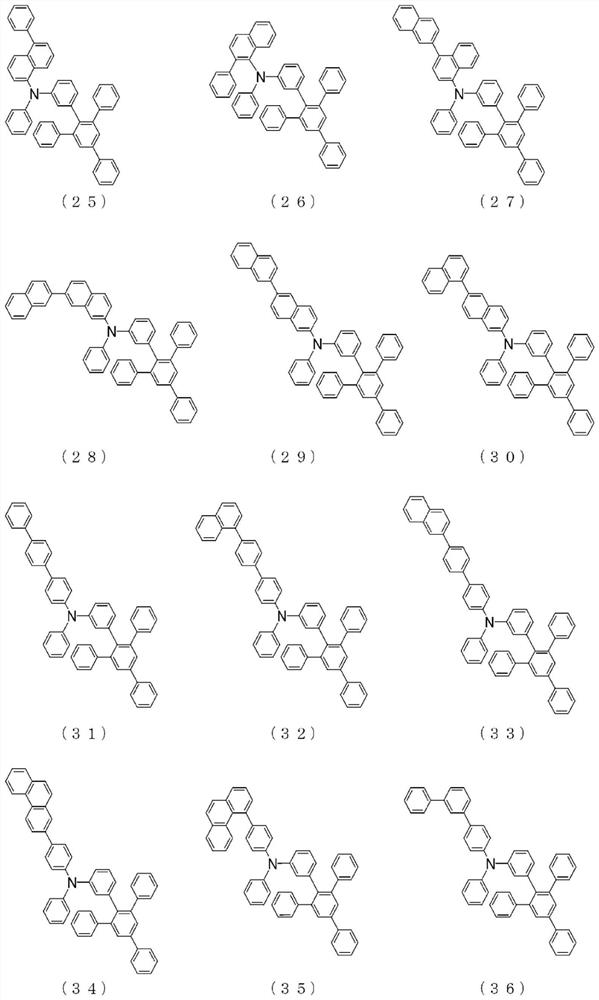
Arylamine compound and organic electroluminescent element
An arylamine compound, electromechanical technology, applied in the field of arylamine compounds and organic electroluminescent elements, can solve the problems of low driving voltage luminous efficiency, unexpectable luminous efficiency, insufficient electron barrier, etc. Excellent, excellent electron blocking ability, good injection characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107]
[0108] Incorporate 2,4,6-triangular-bromoly: 7.0g, double (couplet-4-base)-[3- (4, 4, 5, 5-four-in-four bases- [1 , 3, 2] -The bonitrine-2-base) -Bhenyl] -Anamin: 12.4g, [1, 1'-dual (two phenylbenyl) Ermao Iron] 钯 (II) Dichloride · dichloromethane additive: 0.3g, sodium bicarbonate: 3.6g, in THF / H 2 O mixed solvent will stir the next night. After natural cooling, add ethyl acetate / h to the system 2 O, remove the organic layer through extraction and liquid operation, concentrate, and obtain rough products. By refining the obtained crude products through the column chromatography (carrier: silicone, elute: dichloromethane / orthopedane), obtain double (colon-4-yield)-(3 ', 5'-two benzene base -1,1, 1,: 2, '1 "-triple benzene-3" -Base) -The white powder of amine (compound (58)): 9.5g (income: 74.5 %).
[0109] [化 2]
[0110]
[0111] For the obtained white powder, the NMR identification structure is used.
[0112] use 1 H-nmr (CDCL 3 ) Detect the following 39 hydrogen sig...
Embodiment 2
[0115]
[0116] Incorporate 2,4,6-triangular-bromybenzene: 10.0g, (Lianzhen-4-Ya)-(4-萘 -1-base-phenyl)-[3- (4, 4, 4, 4,5,5-four-meta-[1, 3, 2] -The bonitrine boron-2-base) -Phenyl] -Amine: 22.3g, [1, 1'-double (bignene Bidthyl) Ermao Iron] 二 (II) dichlorophmony · Dichlorimlanide bonus: 0.4g, sodium bicarbonate: 5.1g, in THF / H 2 O mixed solvent will stir the next night. After natural cooling, add ethyl acetate / h to the system 2 O, the organic layer is removed by extraction and liquid operation, and the organic layer is concentrated to obtain crude products. By refining the obtained crude products through the column chromatography (carrier: silicone, elute: dichloromethane / orthopedane), obtain (lin benzene-4-yield)-(3 ', 5'-two phenyl- 1, 1 ': 2', 1 "-triple benzene-3" -Base)-(4-萘 -1-yl-phenyl) -The white powder with amine (compound (59)): 16.0g : 82.0 %).
[0117] [化 3]
[0118]
[0119] For the obtained white powder, the NMR identification structure is used.
[0120] use 1 H-nm...
Embodiment 3
[0123]
[0124] Incorporate 2,4,6-triangular-bromybenzene: 11.0g, (linked benzene-4-yield)-(4-萘 -2-base-phenyl)-[3- (4, 4, 4, 4,5,5-four-in-four bases- [1, 3, 2] -The bonitrine boron-2-base) -Phenyl] -Amine: 24.6g, [1, 1'-double (bignene Bidth) Ermao Iron] 二 (II) dichlorophmony · dichloromethane bonus: 0.5g, sodium bicarbonate: 5.6g, in THF / H 2O mixed solvent will stir the next night. After natural cooling, add ethyl acetate / h to the system 2 O, the organic layer is removed by extraction and liquid operation, and the organic layer is concentrated to obtain crude products. By refining the obtained crude products through the column chromatography (carrier: silicone, elute: dichloromethane / orthopedane), obtain (lin benzene-4-yield)-(3 ', 5'-two phenyl- 1, 1 ': 2', 1 "-trisopene-3" -The)-(4-萘 -2-base-phenyl) -The white powder (compound (60)): 15.5g : 72.0 %).
[0125] [化 4]
[0126]
[0127] For the obtained white powder, the NMR identification structure is used.
[0128] use 1 H-n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com