Diabetes treatment targeting abnormal stem cells
A diabetes and hematopoietic stem cell technology, applied in metabolic diseases, disease diagnosis, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. Insufficient and other problems, to achieve the effect of good prognosis and low load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
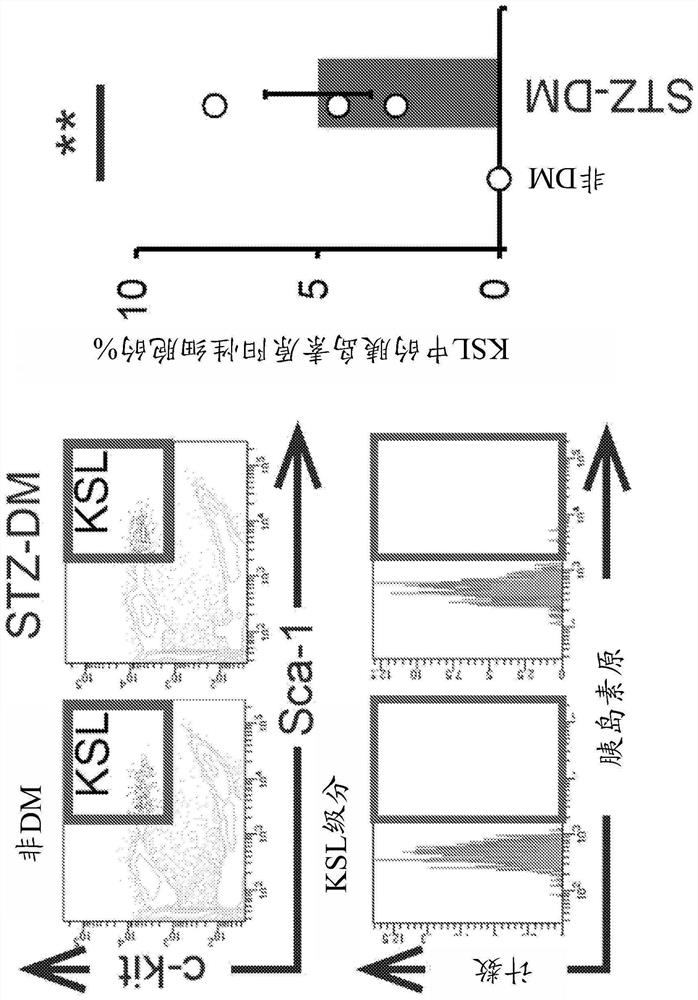
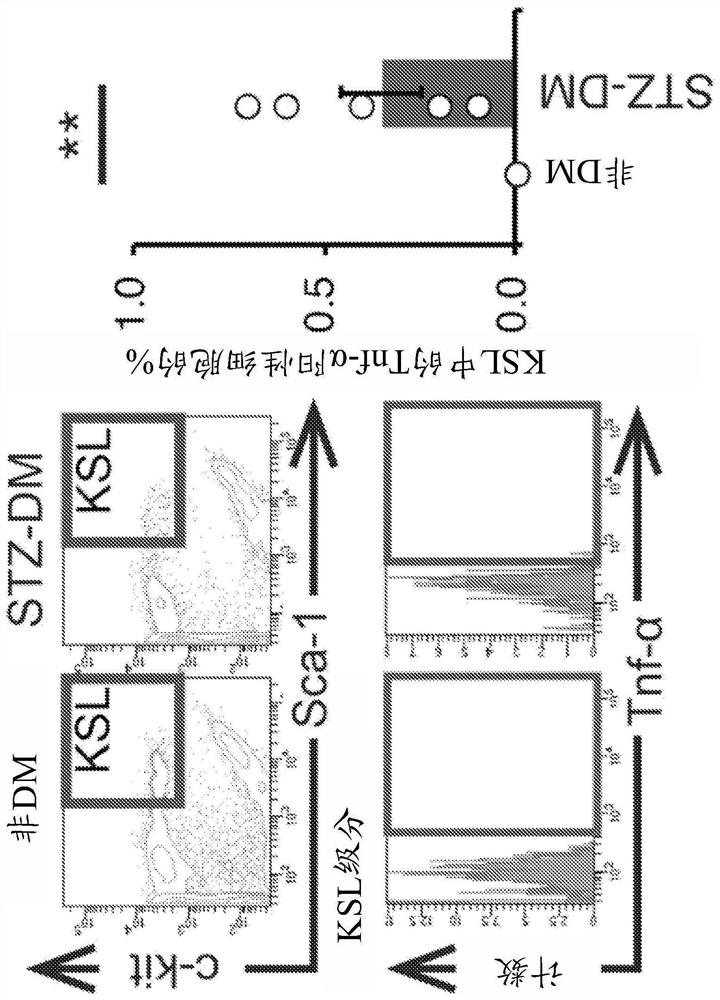
[0245] (Example 1: Identification of abnormal stem cells associated with diabetes)
[0246] To identify abnormal cells associated with diabetes, stem cells obtained from diabetes model mice were analyzed.
[0247] method
[0248] Acquisition of mouse and bone marrow cells
[0249] C57BL / 6J mice (wild type, CLEA, Japan, Osaka) were used in the experiments. Diabetes was induced by intravenous injection of streptozotocin (STZ) (150 mg / kg) (Nakalai Tesque, Kyoto) to create a type 2 diabetes model (STZ mice). Bone marrow cells were isolated from 8-week-old mice.
[0250] FACS
[0251] Mononuclear cells were isolated from whole bone marrow using Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
[0252] To evaluate the expression of TNF-α and CD106, monocytes were stained with PE-Cy7-conjugated streptavidin antibody (BD Biosciences, San Jose, CA), Biotin Mouse Lineage Panel staining (BD Biosciences), APC-conjugated anti-c-kit antibody (BD Biosciences), APC-Cy...
Embodiment 2
[0258] (Example 2: Identification of abnormal stem cells associated with type 1 diabetes)
[0259] In the same manner as in Example 1, the stem cells obtained from the type 1 diabetes model mice were also characterized for abnormal cells related to diabetes.
[0260] method
[0261] Acquisition of mouse and bone marrow cells
[0262] For the test, ICR mice (CLEA, Osaka, Japan) and NOD mice (CLEA, Osaka, Japan) were used. Monocytes were isolated in the same manner as in Example 1.
[0263] As in Example 1, monocytes were stained for TNF-α, CD106, c-kit, Sca-1, and mouse lineage (Lineage), and FACS analysis was performed.
[0264] result
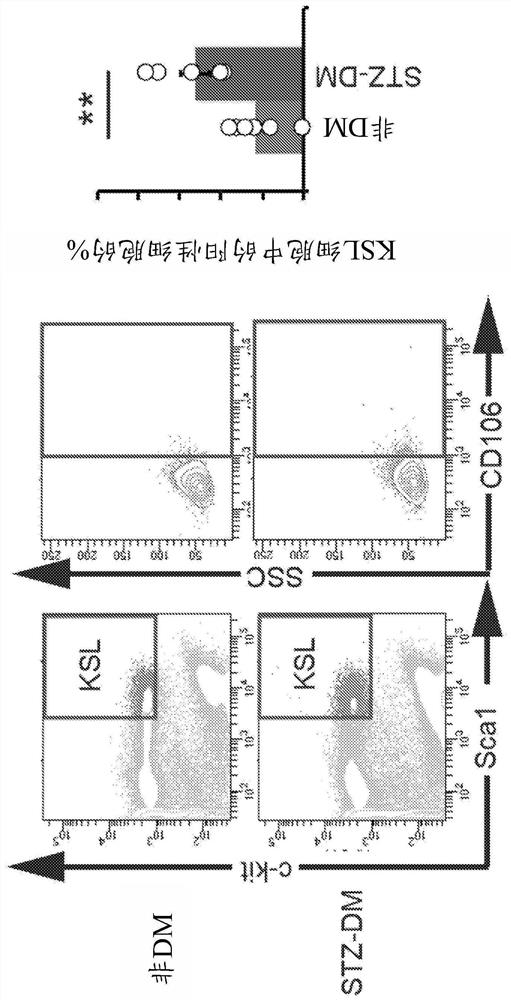
[0265] The proportion of KSL cells was reduced to about 25% in NOD mice compared to ICR mice ( Figure 4 ). The ratios of TNF-α-positive cells and CD106-expressing cells contained in the KSL cell population were significantly increased in NOD mice compared with ICR mice ( Figure 5 ).
[0266] TNF-α-positive cells and CD106-expressing ...
Embodiment 3
[0267] (Example 3: Cell stage of abnormal stem cells associated with diabetes)
[0268] Which stage of the abnormal stem cell is a hematopoietic stem cell is examined.
[0269] method
[0270]Side population cells (SP) of KSL cells were analyzed by FACS as reported by Goodell (cf. J Exp Med. 1996 Apr 1; 183(4): 1797-806; Nat Med. 1997 Dec; 3(12): 1337-45). Hoechest33342 pigment is cell permeable and binds to DNA, however, in hematopoietic stem cells, this pigment can be efficiently excreted outside the cell. Using this property, it was reported that the hematopoietic stem cell fraction was included in the unstained cells when UV light (350 nm) was used to excite bone marrow cells stained with the pigment, and when expanded in two optical filters, Hoechst blue and Hoechst red population (SP cells). Whole bone marrow cells were accurately stained at 37°C for 90 minutes using Hoechest 33342 (Sigma-Aldrich Japan K.K., Tokyo, Japan) as a pigment. To set the gating, Hoechest ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap