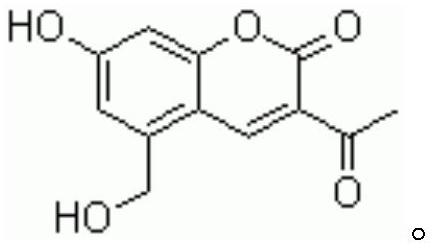
Preparation method of pharmaceutical armillarisin A
A kind of Leupillin A, pharmaceutical grade technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A preparation method of pharmaceutical grade Leupillin A, the specific steps are as follows:
[0033] S1. take 3,5-dihydroxybenzyl alcohol and put it into the reaction flask, add ethanol and stir to dissolve, then add ethyl ethoxymethylene acetoacetate and stir to obtain solution A;
[0034] S2. Weigh sodium metal and dissolve it in ethanol to obtain sodium ethoxide;
[0035] S3. in the solution A obtained in S1, add the sodium ethoxide obtained in S2 to react, leave standstill, and filter to obtain solid A;
[0036] S4. react in S3 to obtain solid A and purified water to dissolve and add activated carbon for decolorization, and filter the activated carbon to obtain filtrate A;
[0037] S5. in the filtrate A that S4 obtains, add dropwise acid to adjust pH, filter to obtain leucovorin A crude product;
[0038] S6. the leucocidin A crude product obtained in S5 is placed in dehydrated alcohol and purified water mixed solution, after adding ammoniacal liquor to dissolve t...
Embodiment 1
[0052] Weigh 100g of 3,5-dihydroxybenzyl alcohol into a 2000mL reaction flask, add 1000g of absolute ethanol and stir until dissolved, add 150g of ethyl ethoxymethylene acetoacetate and stir to reach solution A for 40 minutes.
[0053] Weigh 21 g of metallic sodium and dissolve it in 450 g of anhydrous ethanol to obtain sodium ethoxide, add it to solution A, stir and react for 4 hours, stand for 12 hours and filter to obtain solid A.
[0054] The solid A was dissolved with 3000 g of purified water, 20 g of activated carbon was added for decolorization for 30 minutes, and the activated carbon was filtered to obtain filtrate A.
[0055] Filtrate A was added dropwise with dilute hydrochloric acid (60 g of hydrochloric acid and 60 g of purified water) to adjust the pH to 2. The temperature was lowered to 5°C for crystallization for 1 hour, and the crude leucovorin A was obtained by filtration.
[0056] 1500 g of absolute ethanol and 750 g of purified water were added to the leuco...
Embodiment 2
[0061]Weigh 100g of 3,5-dihydroxybenzyl alcohol into a 2000mL reaction flask, add 1000g of absolute ethanol and stir until dissolved, add 180g of ethyl ethoxymethylene acetoacetate and stir to reach solution A for 40 minutes.
[0062] Weigh 30 g of metallic sodium and dissolve it in 470 g of anhydrous ethanol to obtain sodium ethoxide, add it to solution A, stir and react for 4 hours, stand for 12 hours and filter to obtain solid A.
[0063] The solid A was dissolved with 3000 g of purified water, 20 g of activated carbon was added for decolorization for 30 minutes, and the activated carbon was filtered to obtain filtrate A.
[0064] Filtrate A was added dropwise with dilute hydrochloric acid (60 g of hydrochloric acid and 60 g of purified water) to adjust the pH to 2. The temperature was lowered to 5°C for crystallization for 1 hour, and the crude leucovorin A was obtained by filtration.
[0065] 1500 g of absolute ethanol and 750 g of purified water were added to the leucoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com