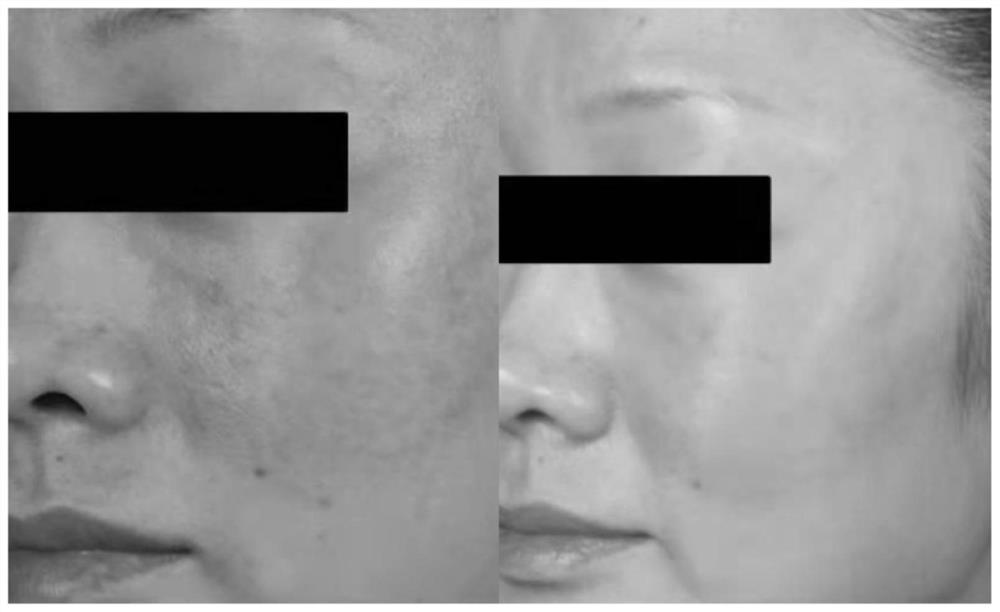
Composite solution with combined application of recombinant III-type humanized collagen and hyaluronic acid and preparation process of composite solution
A collagen and hyaluronic acid technology, applied in the field of medical cosmetology, can solve the problems of high production and testing costs, the product has no biological activity, and the prokaryotic expression system is low-level, and achieves stable product quality, excellent water absorption and moisturizing performance, and high biological active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A complex solution of recombinant type III humanized collagen and hyaluronic acid in combination, comprising the following concentrations of components:
[0041]
[0042]
[0043] The solvent is a physiological buffer.
[0044] The amino acids include glutamic acid, glycine and cysteine in a mass ratio of 1:1:2.
[0045] The physiological buffer solution includes sodium chloride 9 g / L, sodium dihydrogen phosphate 0.07 g / L, disodium hydrogen phosphate 0.2 g / L, and the solvent is water for injection.
[0046] The average molecular weight of the sodium hyaluronate is 1800kDa; the average molecular weight of the recombinant type III humanized collagen is 55kDa.
[0047] The preparation process of the composite solution for the combined application of the recombinant type III humanized collagen and hyaluronic acid includes the following steps:
[0048] S1, take 1g of sodium hyaluronate powder and put it in 50ml of cold water for injection, swell naturally for 60min...
Embodiment 2
[0055] A complex solution of recombinant type III humanized collagen and hyaluronic acid in combination, comprising the following concentrations of components:
[0056]
[0057] The solvent is a physiological buffer.
[0058] The amino acids include glutamic acid, glycine and cysteine in a mass ratio of 1:1:2.
[0059] The physiological buffer solution includes sodium chloride 9 g / L, sodium dihydrogen phosphate 0.07 g / L, disodium hydrogen phosphate 0.2 g / L, and the solvent is water for injection.
[0060] The average molecular weight of the sodium hyaluronate is 2000kDa; the average molecular weight of the recombinant type III humanized collagen is 100kDa.
[0061] The preparation process of the composite solution for the combined application of the recombinant type III humanized collagen and hyaluronic acid includes the following steps:
[0062] S1, take 1g of sodium hyaluronate powder and put it in 50ml of cold water for injection, swell naturally for 60min, heat and ...
Embodiment 3
[0069] A complex solution of recombinant type III humanized collagen and hyaluronic acid in combination, comprising the following concentrations of components:
[0070]
[0071] The solvent is a physiological buffer.
[0072] The physiological buffer solution includes sodium chloride 9 g / L, sodium dihydrogen phosphate 0.07 g / L, disodium hydrogen phosphate 0.2 g / L, and the solvent is water for injection.
[0073] The average molecular weight of the sodium hyaluronate is 1200kDa; the average molecular weight of the recombinant type III humanized collagen is 30kDa.
[0074] The preparation process of the composite solution for the combined application of the recombinant type III humanized collagen and hyaluronic acid includes the following steps:
[0075] S1, take 2g of sodium hyaluronate powder and put it in 50ml of cold water for injection, swell naturally for 60min, heat and stir evenly at 80°C, the stirring speed is 800rpm, and the stirring time is 30min to obtain solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com