Preparation method of dexrazolone
A technology of dexrazoxane and propylenediaminetetraacetic acid, which is applied in the field of medicine, can solve the problems of large batch operation fluctuation, limited industrial application, and uncontrollable product quality, and achieves easy control of process conditions and simple and easy operation of post-processing , The effect of strong process operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of Dexrazoxane
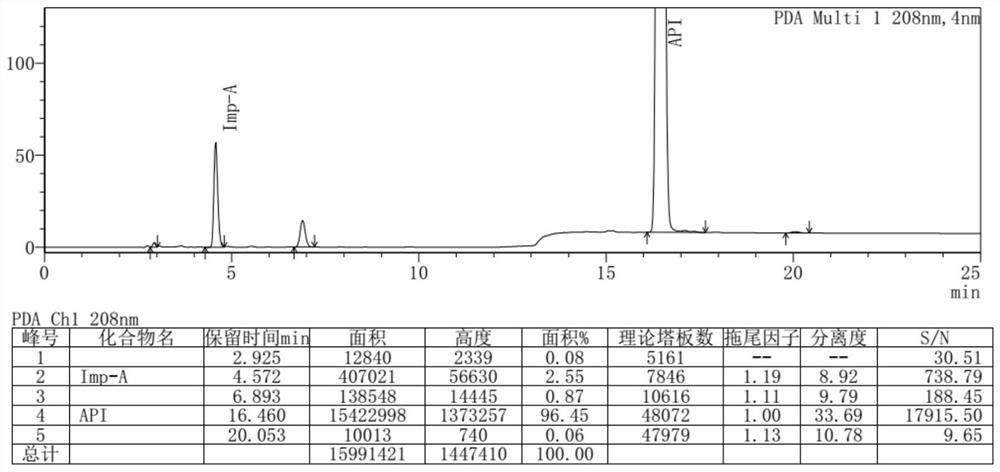
[0059] 15ml of N,N-dimethylformamide, 3.91g of urea, 5.03g of (S)-1,2-propanediaminetetraacetic acid were added to the reaction flask, the temperature was raised to 140°C under stirring, reacted for 5h, and 45ml of ethanol was added at room temperature Stir for 1 h, filter with suction, and dry to obtain 2.89 g of an off-white solid with a yield of 65% and a purity of 96.45% detected by HPLC.
Embodiment 2
[0061] Synthesis of Dexrazoxane
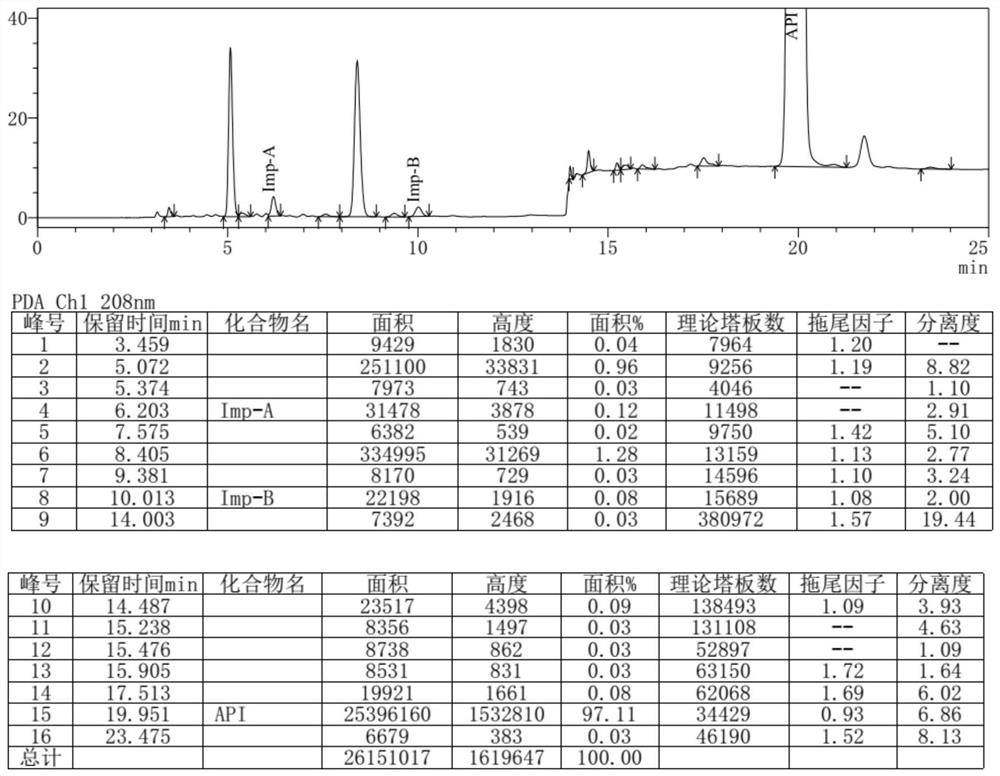
[0062] 15ml of dimethyl sulfoxide, 2.98g of urea, 5.07g of (S)-1,2-propanediaminetetraacetic acid were added to the reaction flask, the temperature was raised to 180°C under stirring, reacted for 3h, 30ml of ethanol was added, stirred at room temperature for 1h, and suction filtered , dried to obtain 3.09g off-white solid, yield 69%, HPLC detection purity 97.11%.
Embodiment 3
[0064] Synthesis of Dexrazoxane
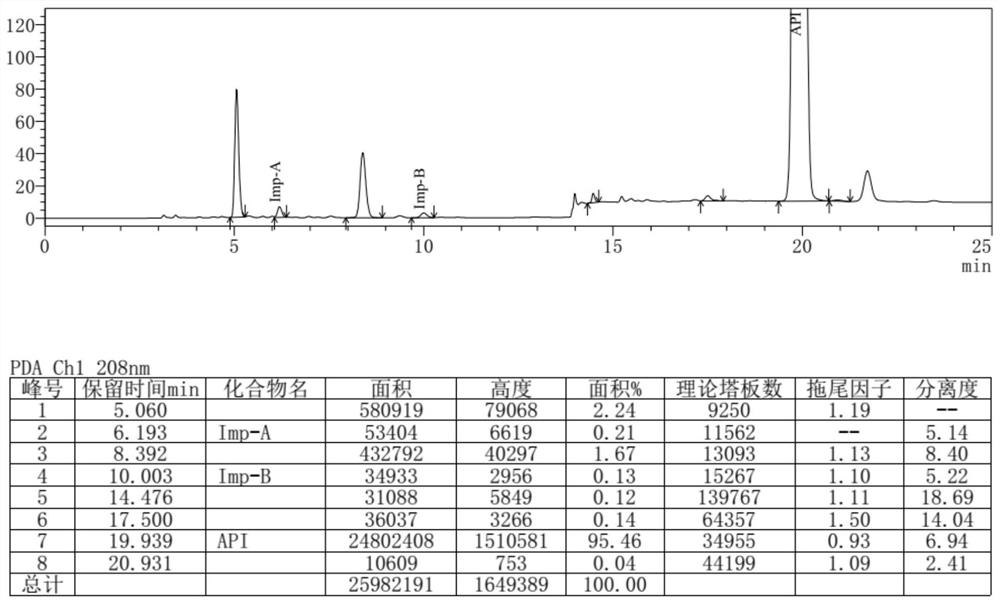
[0065] 15ml of N,N-dimethylacetamide, 2.98g of urea, 5.01g of (S)-1,2-propanediaminetetraacetic acid were added to the reaction flask, the temperature was raised to 160°C under stirring, reacted for 6h, and 105ml of ethanol was added at room temperature Stir for 1 h, filter with suction, and dry to obtain 3.28 g of an off-white solid with a yield of 75% and a purity of 95.46% detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com