Preparation method of melatonin
A technology of melatonin and methoxyl, which is applied in the field of melatonin preparation and can solve the problems of many side reactions, difficult separation of by-products, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
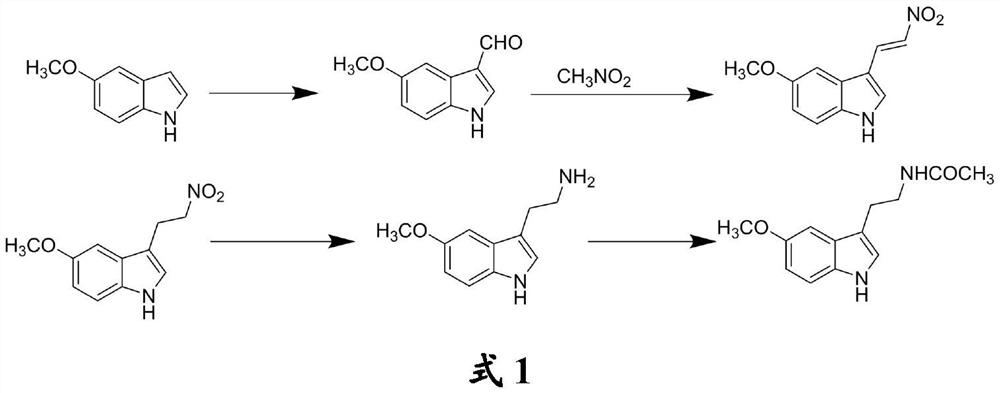
[0026] The invention provides a method for preparing melatonin, comprising the following steps:
[0027] (1) Mix 5-methoxyindole, N,N-dimethylformamide, phosphorus oxychloride and alkaline agent, and carry out Vilsmeier-Haack formylation reaction to obtain 5-methoxyindole-3 -formaldehyde;
[0028] (2) Mix the 5-methoxyindole-3-carbaldehyde, nitromethane and a catalyst, and carry out Henry reaction to obtain 5-methoxyl-3-(2-nitrovinyl)indole;
[0029] (3) the 5-methoxy-3-(2-nitrovinyl) indole, the first reducing agent and the 5-methoxy-3-(2-nitrovinyl) indole and the second A good solvent of a reducing agent is mixed, and a carbon-carbon double bond reduction reaction is carried out to obtain 5-methoxy-3-(2-nitroethyl) indole;
[0030] (4) the 5-methoxy-3-(2-nitroethyl) indole, the second reducing agent and the 5-methoxy-3-(2-nitroethyl) indole and the first The good solvents of the two reducing agents are mixed, and the nitro reduction reaction is carried out under acidic c...
Embodiment 1
[0050] (1) Add 1.5mL phosphorus oxychloride and 10mL DMF to the reactor, and stir at 5°C for 10min; dropwise add 3g of 5-methoxyindole mixed solution dissolved in 20mL DMF solution, and the dropwise addition is completed in 20min. During the dropwise addition, the temperature was controlled at <10°C. After the dropwise addition, the reactor was controlled to stir at 35°C for 1 hour; then 12 g of ice and 30 mL of 6 mol / L sodium hydroxide solution were added to the solution, and heated to reflux at 100°C for 6 hours. TLC monitors the reaction process; after the reaction, add 60mL ethyl acetate to the reactor reaction solution for extraction, then dry the extract phase with 20g of anhydrous sodium sulfate, filter to remove anhydrous sodium sulfate, and finally remove ethyl acetate by rotary evaporation at 35°C ester to give 5-methoxyindole-3-carbaldehyde as a red solid. The yield was 85% and the purity was 98.7%.
[0051] (2) Add 3g 5-methoxyindole-3-carbaldehyde, 50mL nitrometh...
Embodiment 2
[0056] (1) Add 6mL of phosphorus oxychloride and 40mL of DMF to the reactor, stir at 3°C for 20min; dropwise add 12g of 5-methoxyindole mixed solution dissolved in 80mL of DMF solution, dropwise in 60min, drop When the addition time is controlled, the temperature is <10°C; after the dropwise addition, control the reactor at 30°C and stir for 2 hours, then add 45g of ice and 120mL of 5mol / L potassium hydroxide solution to the solution, and then heat and reflux at 90°C for 5h. TLC monitors the reaction process; after the reaction, add 200mL ethyl acetate to the reactor reaction solution for extraction, then dry the extract phase with 80g of anhydrous sodium sulfate, filter to remove anhydrous sodium sulfate, and finally remove ethyl acetate by rotary evaporation at 40°C ester to give 5-methoxyindole-3-carbaldehyde as a red solid. The yield was 86% and the purity was 98.5%.
[0057] (2) Add 6g 5-methoxyindole-3-formaldehyde, 100mL nitromethane and 6g ammonium carbonate to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com