Preparation method of triamcinolone acetonide
A compound and reaction technology, applied in the field of drug synthesis, can solve the problems of long reaction steps, high preparation cost of triamcinolone acetonide, affecting process yield and quality, etc., achieve yield and quality improvement, shorten production cycle, save pressure and cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
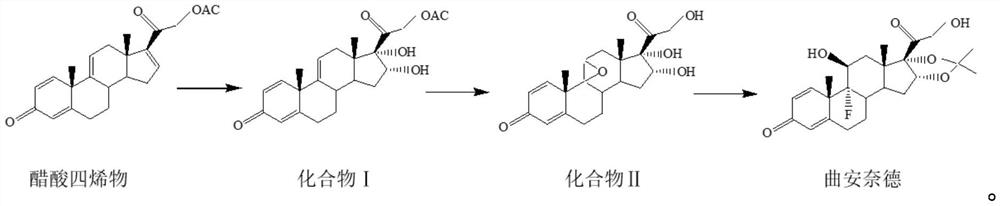
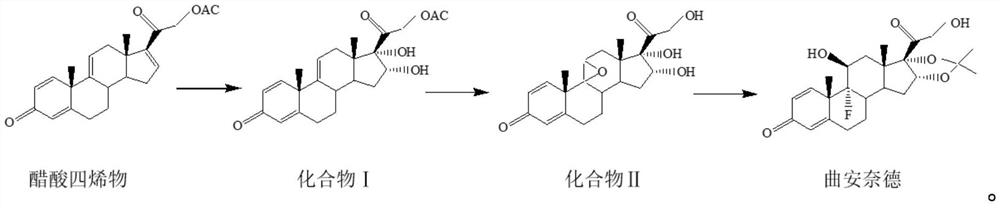
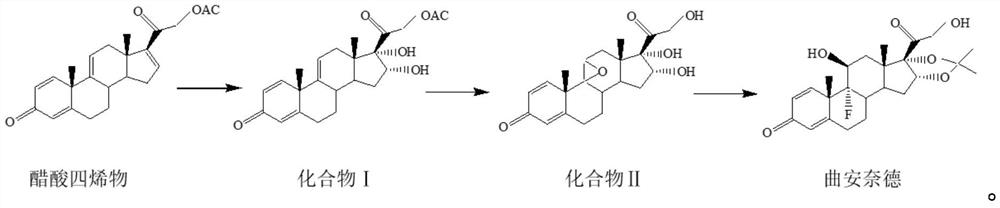
[0039] The preparation method of the triamcinolone acetonide of the present embodiment, reaction scheme is as follows:
[0040]
[0041] S1: Preparation of compound Ⅰ
[0042]Add 50g of tetraenyl acetate and 1500ml of acetone into the reaction flask, cool down to -5°C, add 10g of formic acid, stir for 5min, add potassium permanganate solution (add 25g of potassium permanganate to 400ml of water, stir to dissolve, dissolve Add 300ml of acetone, stir and cool down to -15°C to -20°C for later use), control the temperature at -5°C for 10 minutes, add sodium sulfite solution (add 10g of sodium sulfite to 100ml of water, stir to dissolve), heat up to 40°C, filter, and The filtrate was filtered into a concentration tank, concentrated until there was no acetone, diluted with 1000ml of water, cooled below 10°C, filtered out, and dried at 60°C for 10h to obtain 51g of compound I with a chromatographic purity of 97.9% and a yield of 102%.
[0043] S2: Preparation of compound Ⅱ
[00...
Embodiment 2
[0050] The preparation method of the triamcinolone acetonide of the present embodiment, the steps are as follows:
[0051] S1: Preparation of compound Ⅰ
[0052] Add 50g of tetraenyl acetate and 1500ml of acetone into the reaction flask, cool down to 0°C, add 7.5g of formic acid, stir for 5min, add potassium permanganate solution (add 20g of potassium permanganate to 400ml of water, stir to dissolve, dissolve Add 300ml of acetone, stir and cool down to -15°C to -20°C for later use), control the temperature at 0°C for 5 minutes, add sodium sulfite solution (add 5g of sodium sulfite to 100ml of water, stir to dissolve), heat up to 40°C, filter, and the filtrate Filter into a concentration tank, concentrate to no acetone, add 1000ml of water to dilute, cool down below 10°C, filter and discharge, and dry at 60°C for 10h to obtain 50.5g of compound Ⅰ, chromatographic purity: 98.0%, yield 101%.
[0053] S2: Preparation of compound Ⅱ
[0054] Add 50g of compound I and 800ml of tetr...
Embodiment 3
[0060] The preparation method of the triamcinolone acetonide of the present embodiment, the steps are as follows:
[0061] S1: Preparation of compound Ⅰ
[0062] Add 50g of tetraene acetate and 1500ml of acetone into the reaction flask, cool down to 0°C, add 5g of formic acid, stir for 5min, add potassium permanganate solution (add 30g of potassium permanganate to 400ml of water, stir to dissolve, and dissolve Add 300ml of acetone, stir and cool down to -15°C to -20°C for later use), control the temperature at 0°C for 15 minutes, add sodium sulfite solution (add 15g of sodium sulfite to 100ml of water, stir to dissolve), heat up to 40°C, filter, and filter the filtrate Put it into a concentration tank, concentrate until there is no acetone, add 1000ml of water to dilute, lower the temperature below 10°C, filter out the material, and dry at 60°C for 10h to obtain 50.5g of compound I with a chromatographic purity of 98.2% and a yield of 101%.
[0063] S2: Preparation of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com