Compound containing 1, 3-diketone ligand, application of compound and organic electroluminescent device
A compound and ligand technology, applied in the field of organic electroluminescent devices, can solve the problems of large efficiency roll-off and low luminous efficiency, and achieve the effects of improving lifespan, luminous efficiency and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1-3
[0073] According to particularly preferred embodiments 1-3 , in Ir(L A )(L B ) 2 In the structure shown,
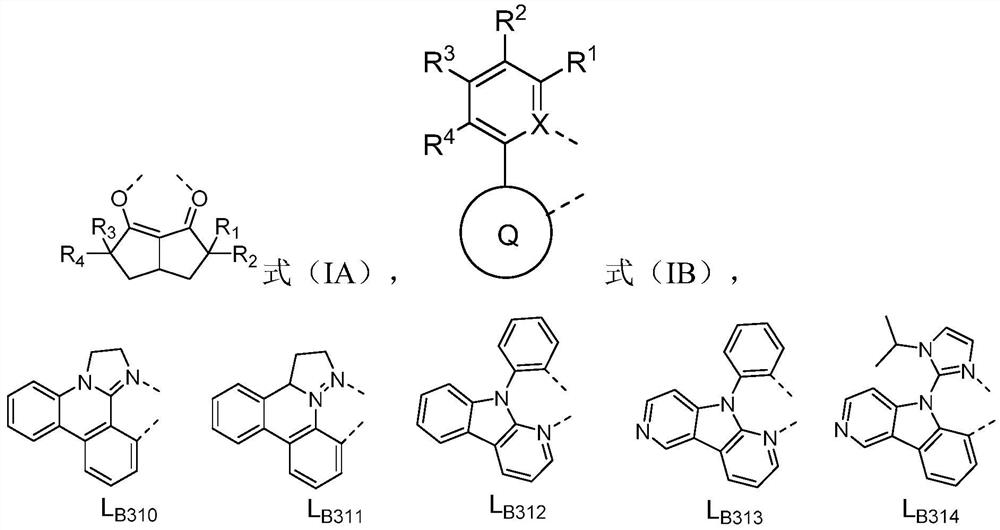
[0074] In formula (IA), R 1 , R 2 , R 3 , R 4 each independently selected from H, C 1 -C 7 Alkyl, C 6 -C 10 Aryl; or R 1 with R 2 combination of and R 3 with R 4 At least one of the combinations of the combinations is ring-closed to form a 4-6 membered saturation;
[0075] In formula (IB), X is C or N,
[0076] Q ring is selected from substituted or unsubstituted benzene ring, substituted or unsubstituted quinoline ring, substituted or unsubstituted isoquinoline ring, substituted or unsubstituted naphthalene ring, substituted or unsubstituted phenanthrene ring, substituted or unsubstituted Substituted benzothiophene ring, substituted or unsubstituted benzofuran ring, substituted or unsubstituted indole ring, substituted or unsubstituted benzothiazole ring, substituted or unsubstituted benzoxazole ring, substituted or unsubstituted Substituted benzimidazo...
specific Embodiment approach 1-4
[0081] According to particularly preferred embodiments 1-4 , in Ir(L A )(L B ) 2 In the structure shown,
[0082] In formula (IA), R 1 , R 2 , R 3 , R 4 each independently selected from H, methyl, ethyl, C 3 straight chain alkyl, C 3 branched chain alkyl, C 3 Cycloalkyl, C 4 straight chain alkyl, C 4 branched chain alkyl, C 4 Cycloalkyl, C 5 straight chain alkyl, C 5 branched chain alkyl, C 5 Cycloalkyl, C 6 straight chain alkyl, C 6 branched chain alkyl, C 6 Cycloalkyl, C 7 straight chain alkyl, C 7 branched chain alkyl, C 7 Cycloalkyl, phenyl; or R 1 with R 2 combination of and R 3 with R 4 At least one combination of the combinations is ring-closed to form a 4-6 membered saturated ring;
[0083] In formula (IB), X is C or N,
[0084] Q ring is selected from substituted or unsubstituted benzene ring, substituted or unsubstituted quinoline ring, substituted or unsubstituted isoquinoline ring, substituted or unsubstituted naphthalene ring, substituted ...
specific Embodiment approach 1-6
[0097] According to particularly preferred embodiments 1-6 , in Ir(L A )(L B ) 2 In the structure shown,
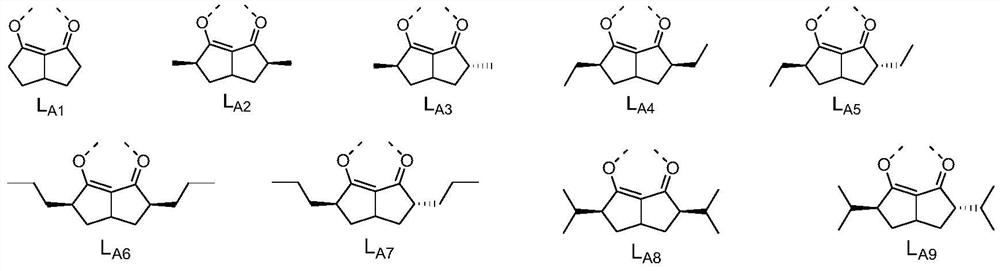
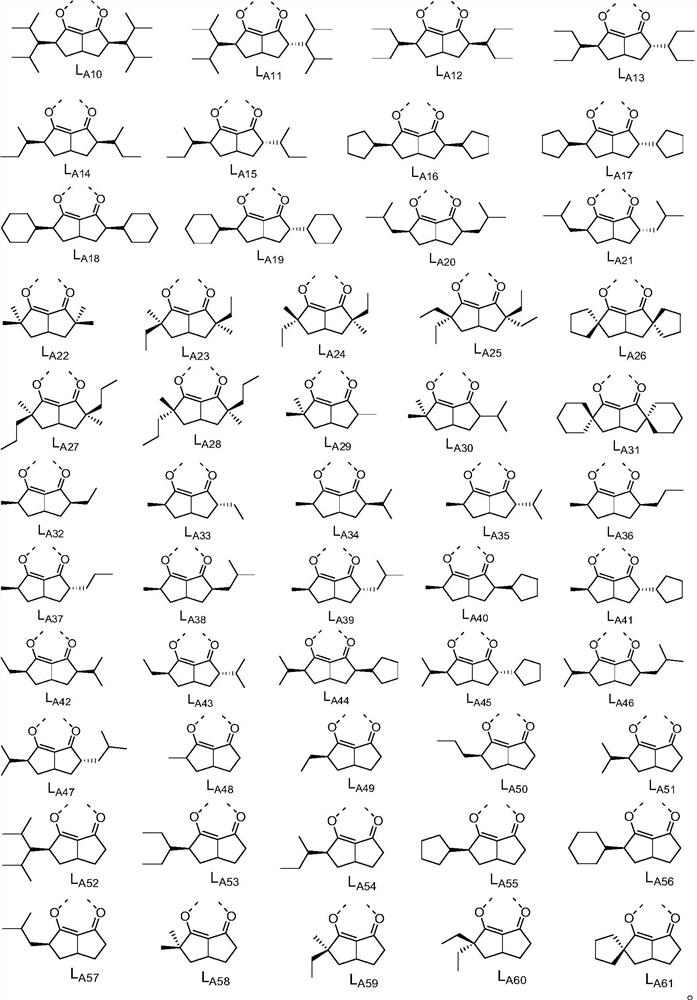
[0098] L A selected from the structures shown in claim 6; and
[0099] L B Selected from the structures shown in claim 7.
[0100] According to preferred embodiments 1-7 , Ir(L A )(L B ) 2 The structure shown is selected from the structures shown in claim 8.
[0101] The present invention has no special limitation on the preparation method of the compound containing 1,3-diketone ligand described in the aforementioned first aspect, and those skilled in the art can determine the appropriate reaction route according to the structural formula combined with known methods in the field of organic synthesis . The following sections of the present invention exemplarily provide several methods for preparing the compounds containing 1,3-diketone ligands described in the first aspect, which should not be understood by those skilled in the art to limit the present invent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com