Staphylococcus aureus TRAP targeted recombinant protein antigen and application thereof
A technology of recombinant protein and staphylococcus, which is applied in the field of biomedicine, can solve the problems of insufficient targeting performance of TRAP protein and low immune level, and achieve the effect of enhancing the level of humoral immune response, reducing the immune dose, and accelerating the popularization of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
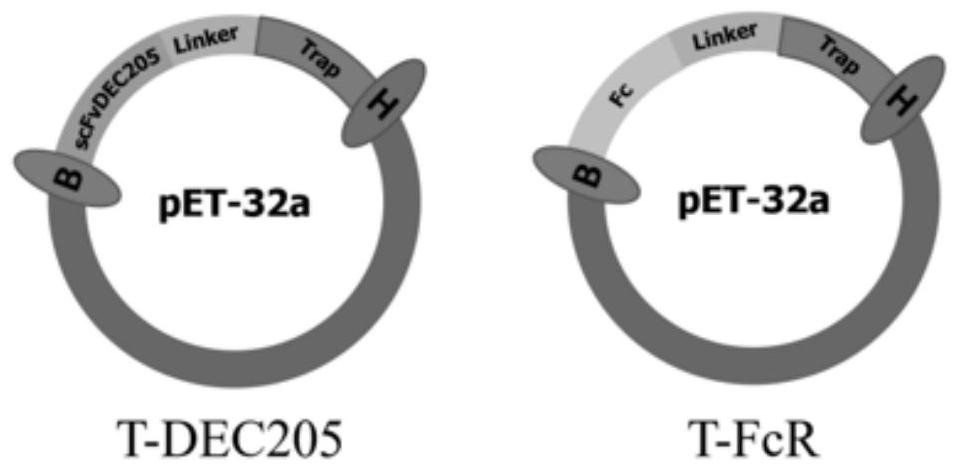
[0042] This example is the construction and prokaryotic expression of TRAP antigen vector targeting DC
[0043] 1. Alignment of mouse and bovine DEC205 receptor protein structures
[0044] The mouse DEC205 receptor structure and Fc receptor structure, the bovine DEC205 receptor structure and Fc receptor structure were found by consulting the literature and NCBI, and through the Blast function of NCBI (https: / / blast.ncbi.nlm.nih. gov / Blast.cgi), compare the primary structure of proteins online, such as figure 2 , whose homology is 85%; use the online comparison website PROMALS3D (http: ∥prodata.swmed.edu / promals3d / promals3d.php), compare the secondary structure of the protein, such as image 3 , their protein folding methods are highly similar; use the RCSBPDB database (http: / / www.rcsb.org / ) and PyMOLWin software to compare the tertiary structure of proteins, such as Figure 4 , the overlap between the two proteins is very high. Such as Figure 5 It can be seen that the ov...
Embodiment 2
[0057] This example illustrates the optimization of T-DEC205 and T-FcR immune conditions
[0058] 1. Antigen Preparation
[0059] Adjust the concentration of purified pET-32a, TRAP, T-DEC205, and T-FcR proteins to 1 mg / mL, 0.5 mg / mL, 0.1 mg / mL, 0.01 mg / mL, and 0.001 mg / mL, respectively. :1 mixed with complete Freund's adjuvant, fully emulsified with an emulsifier and then set aside at 4°C.
[0060] 2. Immunization of Mice
[0061] Eight-week-old female BALB / c mice were randomly divided into 20 groups, with 4 mice in each group. The pET-32a, TRAP, T-DEC205, and T-FcR protein antigens prepared above were injected into the hind leg muscles to immunize the mice for the first time, and the inoculation dose was 100 μL per mouse.
[0062] 3. Isolation of Mouse Spleen Lymphocytes
[0063] Take 3 mice in each group for spleen isolation, prepare 10 mL splenocyte suspension with 1640 medium, add 1 mL red blood cell lysate to resuspend the cell pellet after centrifugation, discard the...
Embodiment 3
[0084] This example illustrates the immune effect of targeting recombinant proteins.
[0085] 1. Immunization of experimental animals
[0086] One hundred 8-week-old female BALB / c mice were randomly divided into four groups. After diluting the protein and mixing it with complete Freund's adjuvant at a volume ratio of 1:1, the mice were first immunized by intramuscular injection in the hind legs, and the inoculation dose was 100 μL per mouse. Three weeks after the initial immunization, the same protein was mixed with incomplete Freund's adjuvant at a volume ratio of 1:1, and the mice were immunized for the second time in the same immunization method. The tagged protein expressed by pET-32a plasmid was mixed with Freund's adjuvant in an equal volume to emulsify and immunize mice as a control.
[0087] 2. Mice challenge
[0088] One week after the booster immunization, different doses of Newman bacteria suspension were used to prechallenge the same batch of unimmunized mice by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com