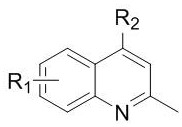
Preparation method of 4-site alkylated derivative of 2-methylquinoline compound
A technology of methyl quinoline and compound is applied in the field of preparation of 4-position alkylated derivatives, which can solve the problem of large amount of carboxylic acid, and achieve the advantages of less side reaction products, good substrate solubility and wide applicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] -Methylquinoline (1 mmol), acetic acid (2.0 mmol), silver nitrate (0.5 mmol), Selectfluor (4 mmol) and magnetons were added to the aqueous solution (10 ml) and reacted at 80 ℃ for 8 h, and 20 ml Add water and 20 ml ethyl acetate, stir rapidly with sodium bicarbonate until no bubbles are generated, extract with ethyl acetate (3*20 mL), combine the organic layers, dry over anhydrous sodium sulfate, filter, and concentrate. Column chromatography (eluent: ethyl acetate / n-hexane = 1:15) yielded 142.8 mg of the product 2,4-dimethylquinoline with a yield of 91%.
[0030] 1 H NMR (400 MHz, CDCl 3 ) δ 8.01 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.3Hz, 1H), 7.69–7.62 (m, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.11 (s, 1H), 2.68 (s,3H), 2.64 (s, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ 158.56 , 147.56 , 144.20 , 129.07, 129.01 , 126.49 , 125.37 , 123.51 , 122.64 , 25.10 , 18.50 .
Embodiment 2
[0032] 2-Methylquinoline (1 mmol), propionic acid (2.0 mmol), silver nitrate (0.5 mmol), Selectfluor (4 mmol) and magnetons were added to aqueous solution (10 ml) and reacted at 80 °C for 8 h, adding Add 20 ml of water and 20 ml of ethyl acetate, add sodium bicarbonate and stir quickly until no bubbles are generated, extract with ethyl acetate (3*20 mL), combine the organic layers, dry over anhydrous sodium sulfate, filter, and concentrate. Column chromatography (eluent: ethyl acetate / n-hexane = 1:15) yielded 153.5 mg of the product 4-ethyl-2-methylquinoline with a yield of 90%.
[0033] 1 H NMR (400 MHz, CDCl 3 ) δ 8.02 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 8.3Hz, 1H), 7.65–7.60 (m, 1H), 7.48–7.43 (m, 1H), 7.10 (s, 1H), 3.03 (q, J = 7.5Hz, 2H), 2.69 (s, 3H), 1.35 (t, J = 7.5 Hz, 3H). 13 C NMR (100 MHz, CDCl 3 ) δ158.66 , 149.72 , 147.77 , 129.18 , 128.85 , 125.60 , 125.28 , 123.08 ,120.51 , 25.20 , 24.82 , 13.92 .
Embodiment 3
[0035] 2-Methylquinoline (1 mmol), isobutyric acid (2.0 mmol), silver nitrate (0.5 mmol), Selectfluor (4 mmol) and magnetons were added to aqueous solution (10 ml) and reacted at 80 °C for 8 h, adding Add 20 ml of water and 20 ml of ethyl acetate, add sodium bicarbonate and stir quickly until no bubbles are generated, extract with ethyl acetate (3*20 mL), combine the organic layers, dry over anhydrous sodium sulfate, filter, and concentrate. Column chromatography (eluent: ethyl acetate / n-hexane = 1:15) yielded 162.9 mg of the product 4-isopropyl-2-methylquinoline with a yield of 88%.
[0036] 1 H NMR (600 MHz, CDCl 3 ) δ 8.02 (t, J = 8.8 Hz, 2H), 7.63 (t, J = 7.6Hz, 1H), 7.47 (t, J = 7.6 Hz, 1H), 7.17 (s, 1H), 3.67 (dd, J = 13.7, 6.9 Hz,1H), 2.71 (s, 3H), 1.37 (s, 3H), 1.36 (s, 3H). 13 C NMR (150 MHz, CDCl 3 ) δ158.71 , 154.16 , 147.98 , 129.38 , 128.69 , 125.22 , 125.04 , 122.79 ,117.63 , 28.09 , 25.41 , 22.80 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com