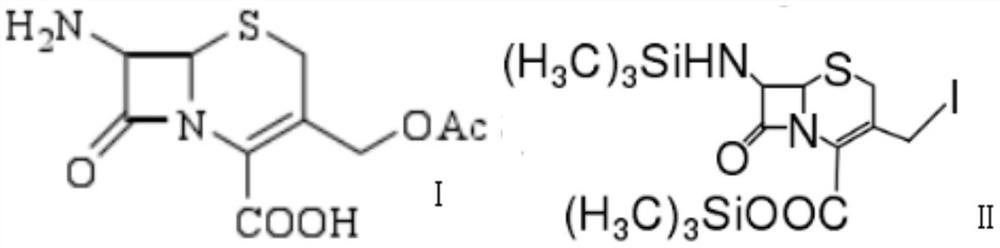Preparation method of cefozopran hydrochloride intermediate
A technology of cefazolam hydrochloride and cefazolam, which is applied in the field of preparation of cefazolam hydrochloride intermediates, can solve problems such as poor stability, decreased purity, easy hydrolysis, etc., and achieves the advantages of shortening reaction time, reducing by-products and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of embodiment 1 cefozopran hydrochloride intermediate
[0028] (1) Using 7-ACA as the starting material, mix and react 7-ACA, hexamethyldisilazane and iodotrimethylsilane at a mass ratio of 1:1.7:0.6 for 2.5 hours at a reaction temperature of 23°C to prepare Compound 1 was obtained.
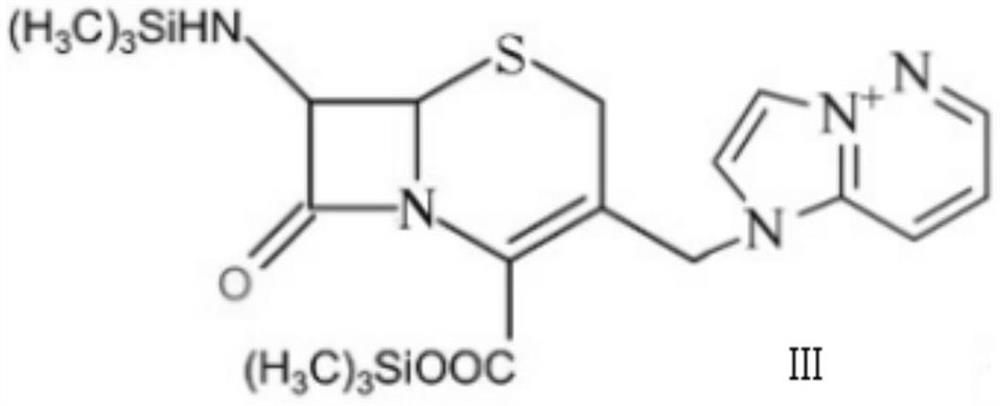
[0029] (2) Compound 1 and imidazo[1,2-b]pyridazine with a mass ratio of 1:2 are mixed with a solvent, the volume ratio of the compound 1 and the solvent is 1:15, and the volume ratio of the solvent is 1 : 2 mixed solution of dimethylformamide and dichloromethane, reacted for 3.5h, and the reaction temperature was 23°C to obtain compound 2.
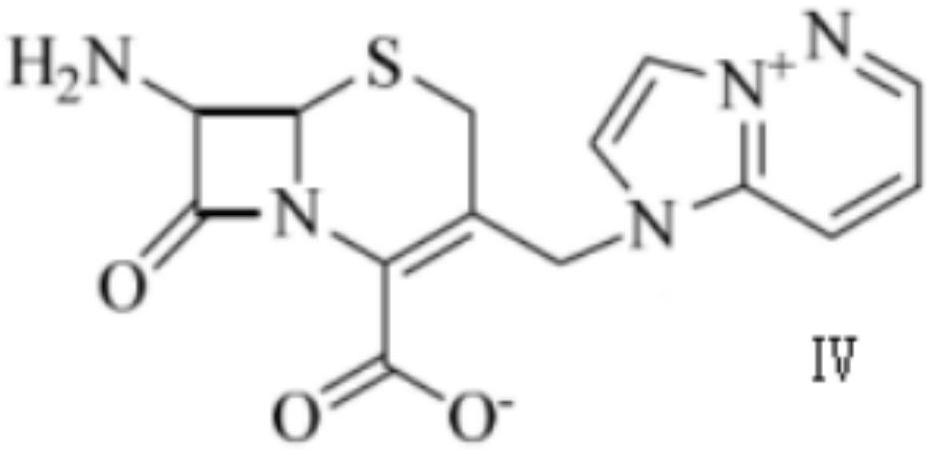
[0030] (3) preparation mass concentration is 6% phosphoric acid acetonitrile solution, compound 2 is added in phosphoric acid acetonitrile solution and reacts 50min, and reaction temperature is 23 ℃, and described compound 2 and phosphoric acid acetonitrile solution volume ratio are 1:5, make ceftizole Blue intermediate crude. ...
Embodiment 2
[0032] The preparation method of embodiment 2 cefozopran hydrochloride intermediate
[0033] (1) Using 7-ACA as the starting material, mix and react 7-ACA, hexamethyldisilazane and iodotrimethylsilane at a mass ratio of 1:1.6:0.5 for 2 hours at a reaction temperature of 20°C to obtain Compound 1.
[0034] (2) Compound 1 and imidazo[1,2-b]pyridazine with a mass ratio of 1:1.5 are mixed with a solvent, the volume ratio of the compound 1 and the solvent is 1:10, and the volume ratio of the solvent is 1 : 2 mixed solution of dimethylformamide and dichloromethane, reacted for 3h, and the reaction temperature was 20°C to obtain compound 2.
[0035] (3) preparation mass concentration is 5% phosphoric acid acetonitrile solution, compound 2 is added in phosphoric acid acetonitrile solution and reacts 40min, and reaction temperature is 20 ℃, and described compound 2 and phosphoric acid acetonitrile solution volume ratio are 1:4, make ceftizole Blue intermediate crude.
[0036] (4) Th...
Embodiment 3
[0037] The preparation method of embodiment 3 cefozopran hydrochloride intermediate
[0038] (1) Using 7-ACA as the starting material, mix 7-ACA, hexamethyldisilazane and iodotrimethylsilane at a mass ratio of 1:1.8:0.7 for 3 hours at a reaction temperature of 25°C to obtain Compound 1.
[0039] (2) Compound 1 and imidazo[1,2-b]pyridazine with a mass ratio of 1:2.5 are mixed with a solvent, the volume ratio of the compound 1 and the solvent is 1:20, and the volume ratio of the solvent is 1 : 2 mixed solution of dimethylformamide and dichloromethane, reacted for 4h, and the reaction temperature was 25°C to obtain compound 2.
[0040] (3) preparation mass concentration is 7% phosphoric acid acetonitrile solution, compound 2 is added in phosphoric acid acetonitrile solution and reacts 60min, and reaction temperature is 25 ℃, and described compound 2 and phosphoric acid acetonitrile solution volume ratio are 1:6, make ceftizole Blue intermediate crude.
[0041] (4) The crude pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com