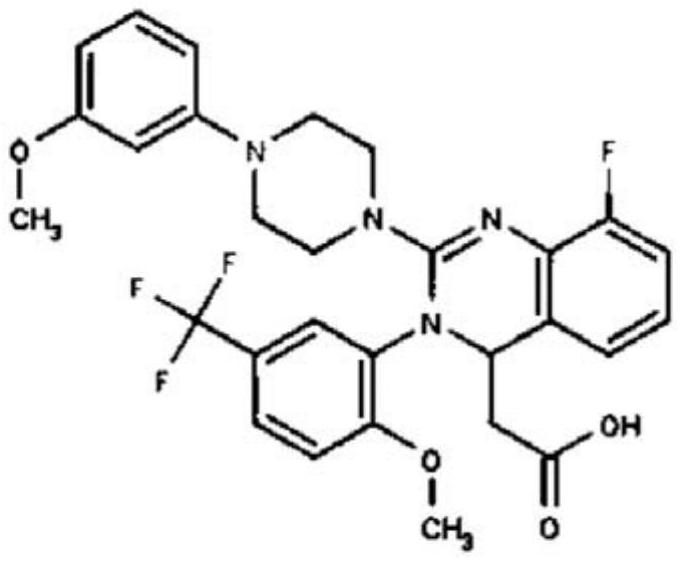
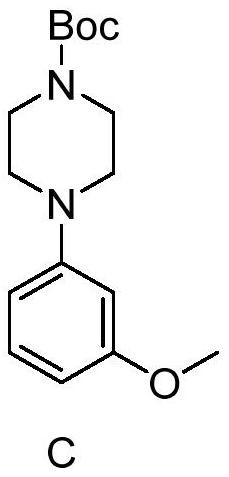
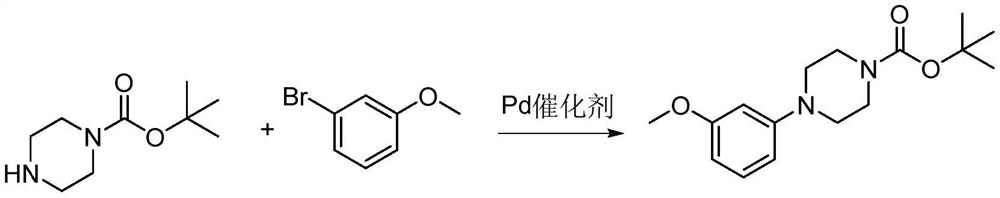
Method for synthesizing letemovir intermediate
A synthesis method and intermediate technology, applied in the field of organic chemical synthesis, can solve the problems of being unsuitable for industrial scale-up production, high potential safety hazards and high production costs, and achieve the effects of low material cost, better effect and easy production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1), add 4-bromoanisole (0.5g), 1-tert-butoxycarbonylpiperazine (1.2eq, 0.6g) in the reaction flask
[0025] and DMSO (3mL);
[0026] 2), then add potassium tert-butoxide (1.5eq, 0.45g);
[0027] 3), heated to 115°C under nitrogen protection for 20h;
[0028] 4), the purity of the reaction solution monitored by HPLC was 61.87%.
Embodiment 2
[0042] Using the organic solvent as a single variable, adopt the method in Example 1 to prepare the Letermovir intermediate, and finally measure and calculate the reaction conversion rate, and the results obtained are as follows:
[0043]
[0044] From the results, the best organic solvent is DMSO, which has a higher conversion rate than tetrahydrofuran and isopropanol.
[0045] In addition, it is found through experiments that the stirring effect of the solvent with an organic solvent dosage of 2-4 vol is poor; the effect of 6-10 vol is better.
Embodiment 3
[0047] With alkali as a single variable, the method in Example 1 is used to prepare the Letermovir intermediate, and finally the reaction conversion rate is measured and calculated, and the results obtained are as follows:
[0048]
[0049]
[0050] It can be seen that the best strong base is potassium tert-butoxide. Although n-butyllithium is more basic than potassium tert-butoxide, its nucleophilicity may be unfavorable to the reaction, but even if its nucleophilicity may be unfavorable to the reaction, Under the condition of not adding copper catalyst, the conversion rate is also higher than the upper limit value of 26% with copper catalyst.
[0051] In addition, comparing the n-butyllithium+copper catalyst method used in Comparative Example 1 with the n-butyllithium used in this example, it can be seen that the copper catalyst is not conducive to conversion.
[0052] The consumption of different alkalis, the conversion rate that obtains is as follows:
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com