Chimeric antigen receptor combined anti-tumor medicine composition and application thereof
An anti-tumor drug and tumor drug technology, applied in the field of biomedicine, can solve the problems of off-target, limited therapeutic effect, poor therapeutic effect, etc., and achieve the effect of improving tumor killing efficiency and significant effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
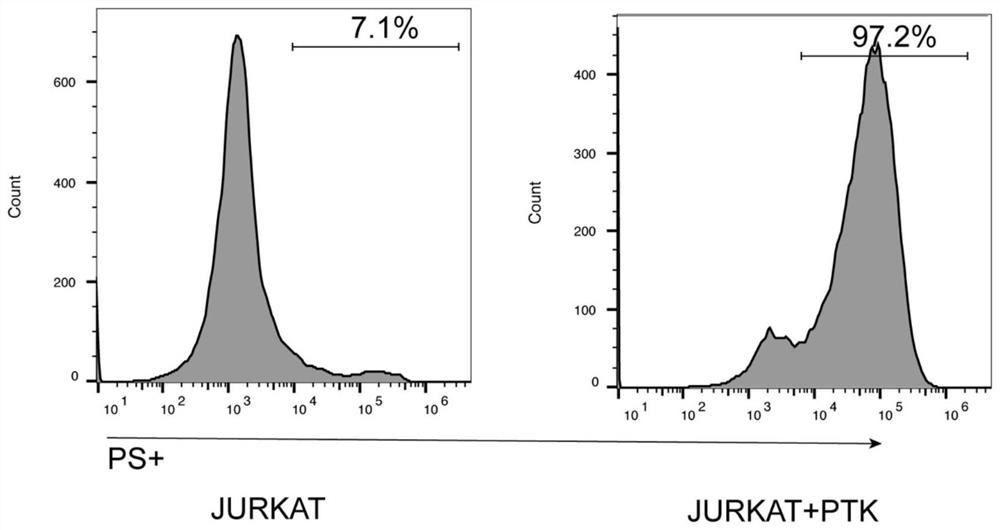
[0030] Example 1 Anti-PS ScFv and protein selection targeting PS
[0031] In order to select a suitable protein structure that can target PS, we selected two ScFvs of two known antibodies that have entered clinical trials, named A1 (US_2016_0015826_A1) and A2 (US_2018_0289771_A1) respectively. For the comparison of the combined non-antibody protein sequences, the most common AnnexinA5 protein was selected and named A3. The amino acid sequence of A1 is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.2; the amino acid sequence of A2 is shown in SEQ ID NO.3, and the nucleotide sequence is shown in SEQ ID NO.4 shown; the amino acid sequence of A3 is shown in SEQ ID NO.5, and the nucleotide sequence is shown in SEQ ID NO.6. The survey results showed that the three anti-PS scFvs or proteins all had high affinity and could be used for the subsequent construction of CAR-T cells.
Embodiment 2
[0032] The construction of embodiment 2 plasmid
[0033] 1. Fragments A1, A2, and A3 were artificially synthesized, and PS, StrepII+G4S-CD8 hinge-CD28TM+ICD-4-1BB-CD3ζ fragments were artificially synthesized.
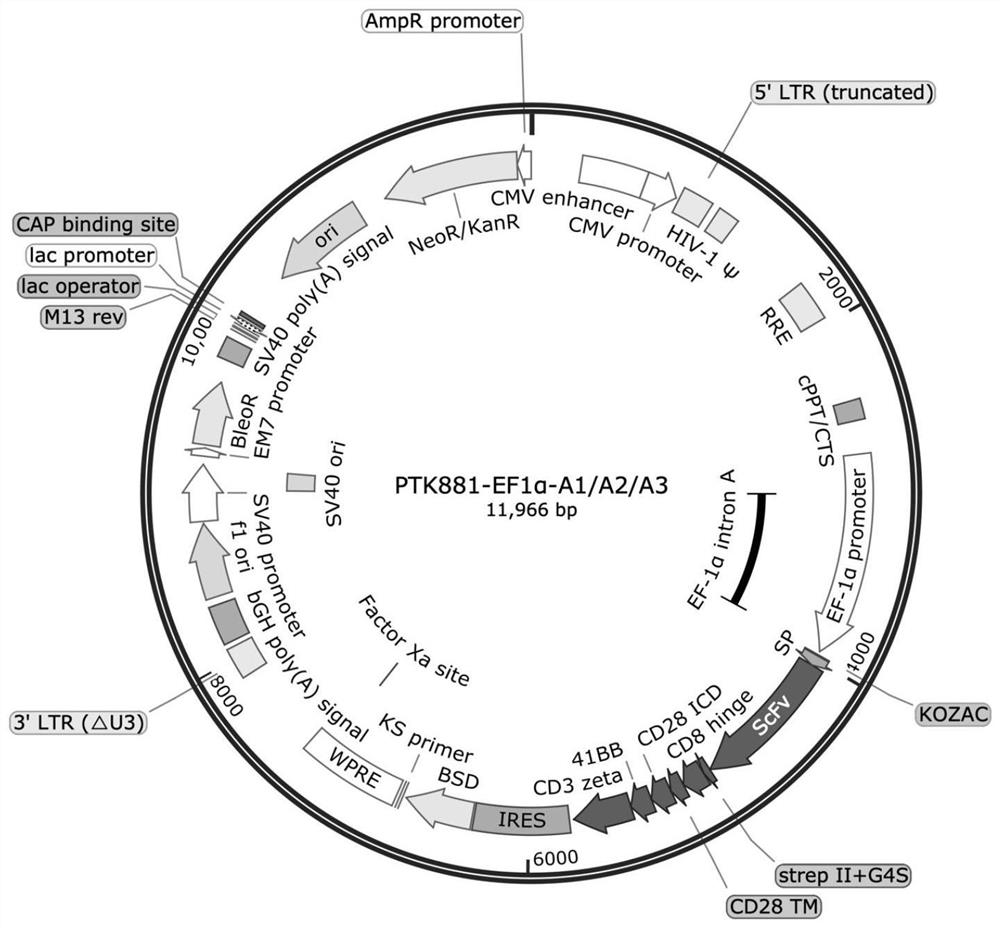
[0034] 2. Using Overlap PCR amplification, PS, A1 / A2 / A3 and StrepII+G4S-CD8 hinge-CD28TM+ICD-4-1BB-CD3ζ were used as templates to obtain A1 with restriction sites EcoR I and BamH I -Structure diagram of CAR, A2-CAR, A3-CAR, A1-CAR, A2-CAR, A3-CAR figure 1 shown;
[0035]Wherein, the amino acid sequence of the signal peptide (PS) is shown in SEQ ID NO.7; the nucleotide sequence is shown in SEQ ID NO.8; the amino acid sequence of StrepII+G4S is shown in SEQ ID NO.9, and the nucleotide The sequence is shown in SEQ ID NO.10; the amino acid sequence of CD8 hinge is shown in SEQ ID NO.11, and the nucleotide sequence is shown in SEQ ID NO.12; the amino acid sequence of CD28TM is shown in SEQ ID NO.13, nucleoside The acid sequence is shown in SEQ ID NO.14; the amino acid seq...
Embodiment 3
[0039] Example 3 Preparation and sequencing of plasmids
[0040] 1. Preparation of plasmid
[0041] Inoculate Escherichia coli DH5α strains containing plasmids PTK881-EF1α-A1, PTK881-EF1α-A2, PTK881-EF1α-A3, respectively into 250mL LB culture medium containing 100μg / mL ampicillin, and culture overnight at 37°C and 220rpm . The culture solution was centrifuged at 6000g for 20min at 4°C, and the supernatant was discarded.
[0042] Take out Buffers P1 from the EndoFree plasma mega kit (Qiagen), add 120mL pre-cooled Buffers P1 to the centrifuged E. coli pellet, cover the cap of the centrifuge bottle, shake the centrifuge bottle vigorously to completely disperse the E. coli pellet in Buffers P1 .
[0043] Add 120mL Buffers P2 to the centrifuge bottle, put the cap on the roller mixer, slowly increase the speed to 50rpm, mix thoroughly and place at room temperature for 5min.
[0044] Add 120mL Buffers P3 to the centrifuge bottle, put the cap on the roller mixer, slowly increase t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com