Chimeric antigen receptor taking IFNGR1 as target spot and application of chimeric antigen receptor
An antigen-receptor technology, applied in the field of medicine and biology, can solve the problems of off-target, singleness, and broad-spectrum limitation of treatment, and achieve the effect of improving tumor killing efficiency and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Affinity determination of scFv against IFNGR1
[0034] Anti-IFNGR1 scFvs with strong specificity and high affinity were screened from the large-capacity IFNGR1 phage antibody library prepared with the extracellular domain of IFNGR1 as antigen. Named as B1, B2 and B3, the sequence analysis of their sequences shows that the nucleotide sequences of B1, B2 and B3 are shown in SEQ ID NO: 2, SEQ ID NO: 4 and SEQ ID NO: 6, respectively, and their amino acids are shown in The sequences are shown in SEQ ID NO: 1, SEQ ID NO: 3 and SEQ ID NO: 5, respectively.
[0035] In order to determine the affinity of the above scFv to the antigen IFNGR1, the binding kinetics of the soluble scFv and the extracellular domain of IFNGR1 was further analyzed by surface plasmon resonance analysis, and the Kd values of purified B1, B2 and B3 were calculated. The steps are briefly described as follows:
[0036] According to the nucleotide sequences shown in SEQ ID NO: 2, SEQ ID NO: 4, a...
Embodiment 2
[0037] Example 2: Construction of PTK881-EF1α-B1, PTK881-EF1α-B2, PTK881-EF1α-B3, PTK881-EF1α-B1-7x19, PTK881-EF1α-B2-7x19, PTK881-EF1α-B3-7x19 Plasmids
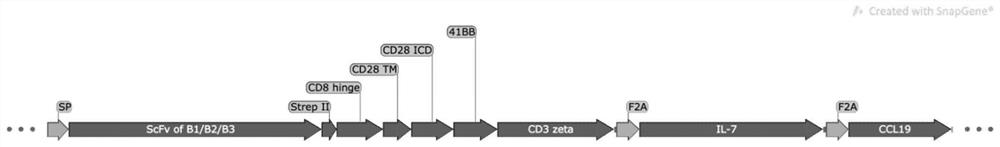
[0038] 1. Artificially synthesize fragments B1, B2, B3, and artificially synthesize SP, strepII-CD8hinge-CD28TM+ICD-4-1BB-CD3ζ fragment.
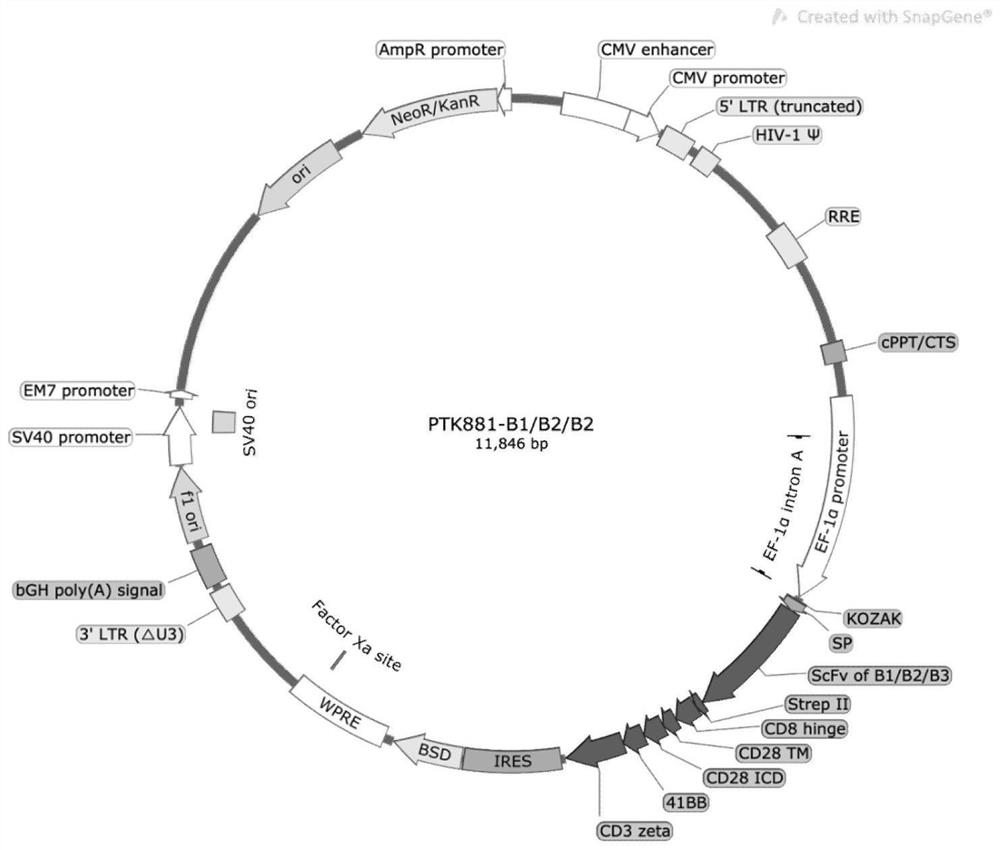
[0039] 2. Use Overlap PCR to amplify with SP, B1 / B2 / B3 and strepII-CD8 hinge-CD28TM+ICD-4-1BB-CD3ζ, or with SP, B1 / B2 / B3 and strepII-CD8 hinge-CD28TM+ICD -4-1BB-CD3ζ+F2A peptide+IL-7+F2A peptide+CCL19 as template to obtain B1-CAR, B2-CAR, B3-CAR, B1-7x19 CAR with restriction sites EcoR I and BamH I , B2-7x19 CAR, B3-7x19 CAR, the schematic diagram of the structure of B1-CAR, B2-CAR, B3-CAR figure 1 shown; the structural schematic diagrams of B1-7x19 CAR, B2-7x19 CAR, B3-7x19 CAR fragments are shown in figure 2 shown.
[0040] The amino acid sequence of signal peptide (SP) is shown in SEQ ID NO.7, the amino acid sequence of strepII is shown in SEQ ID NO.9, the amino acid sequence of CD...
Embodiment 3
[0044] Example 3. Preparation and sequencing of plasmids
[0045] 1. Plasmid preparation
[0046] Escherichia coli DH5α strains containing plasmids PTK881-EF1α-B1, PTK881-EF1α-B2, PTK881-EF1α-B3, PTK881-EF1α-B1-7x19, PTK881-EF1α-B2-7x19, PTK881-EF1α-B3-7x19 They were inoculated into 250 mL of LB medium containing 100 μg / mL ampicillin, and cultured overnight at 37°C and 220 rpm. The culture medium was centrifuged at 6000g for 20min at 4°C, and the supernatant was discarded.
[0047] Take out Buffers P1 in the EndoFree plasmid mega kit (Qiagen), add 120 mL of pre-cooled Buffers P1 to the E. coli pellet obtained by centrifugation, cover the centrifuge bottle, and shake the centrifuge bottle vigorously to completely disperse the E. coli pellet in Buffers P1 .
[0048] Add 120 mL of Buffers P2 to the centrifuge bottle, cover the bottle and place it on a roller mixer, slowly increase the speed to 50 rpm, mix thoroughly and place at room temperature for 5 minutes.
[0049] Add 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com