Application of Albumin in inhibition of abnormal aggregation of Tau protein
A protein and abnormal technology, applied in the field of biomedicine, can solve problems such as unsatisfactory drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
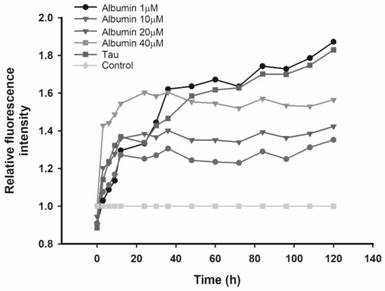
[0039] Example 1: Inhibitory effect of different concentrations of Albumin on Tau R3 protein fibrosis.
[0040] Because heparin sodium induces Tau R3 protein to self-aggregate to form fibers. In order to simulate the abnormal aggregation of Tau protein in the brain to form neurofibrillary tangles, in this example, Tau R3 protein was induced by heparin sodium to aggregate abnormally to form neurofibrillary tangles. Thioflavin T (ThT) can interact with the β-sheet in the aggregated filaments to generate fluorescence, and its fluorescence intensity is positively correlated with the amount of fiber, which can be used for quantitative analysis of protein fiber amount.
[0041] Method: The purchased Tau R3 protein freeze-dried product was precisely weighed, dissolved in PBS, and prepared into a protein solution with a concentration of 1 mM, then diluted to 20 μM with PBS, and the exact concentration was recorded. Accurately weigh Albumin, prepare high-concentration drug mother solu...
Embodiment 2
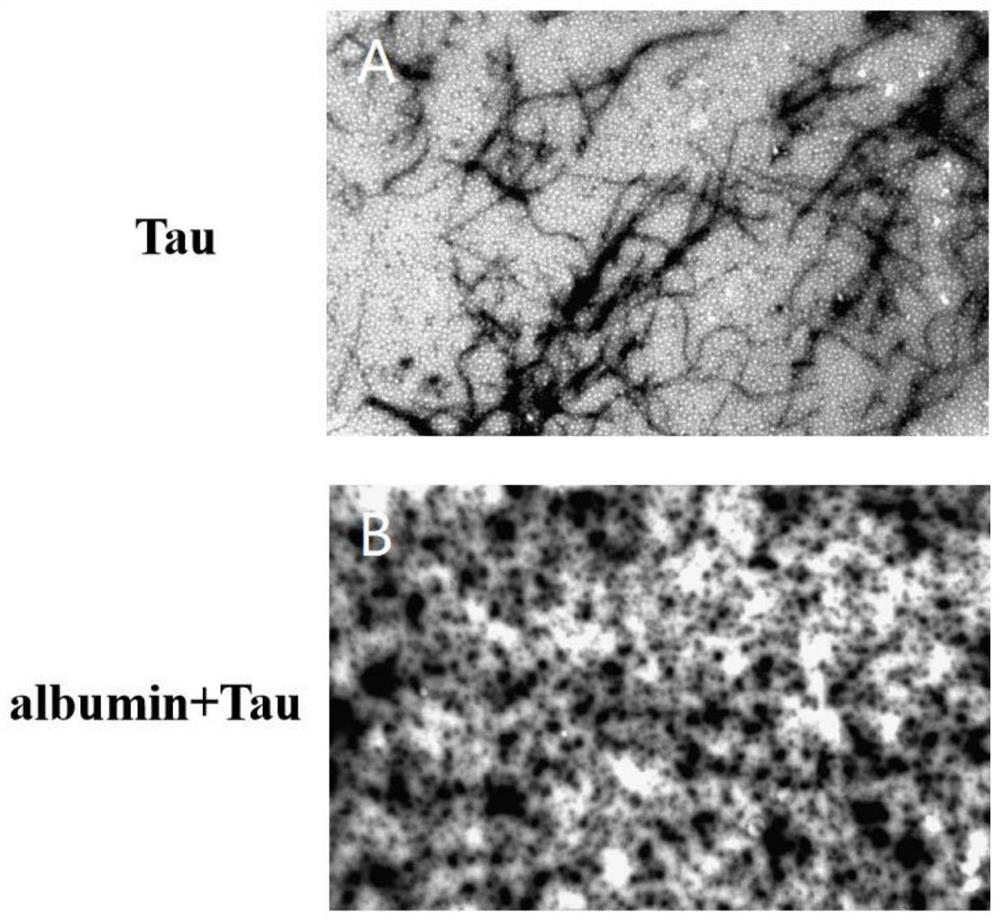
[0049] Example 2: Transmission electron microscopy experiment of Albumin inhibiting Tau R3 protein fibrosis.
[0050] Methods: The aggregation and fibrosis of Tau R3 treated with Albumin can be visually observed by transmission electron microscope (TEM), which is a strong evidence for the ThT fluorescence experiment. From the ThT experiment in Example 1, collect 10 μL of samples from the control group, Albumin group, and TauR3 group respectively, drop them on the copper grid, settle for 1 min, then absorb the excess samples with filter paper, and then drop 2% uranyl acetate for staining , let stand for 5 minutes, then absorb excess uranyl acetate with filter paper, and let stand for 30 minutes. Place in a transmission electron microscope (JEOL, JEM-1011), observe at an accelerating voltage of 100kV, take pictures and save the images.
[0051] Results: The transmission electron microscope pictures are as follows figure 2 As shown, among them, figure 2 -A is the control gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com