Method for preparing hydrogen peroxide by using covalent organic framework catalyst to catalyze oxygen reduction
A covalent organic framework and catalyst technology, applied in the field of electrocatalysis, can solve problems such as precise regulation of catalytic sites, achieve diverse designability, enrich heteroatom catalytic active sites, and improve catalytic performance and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
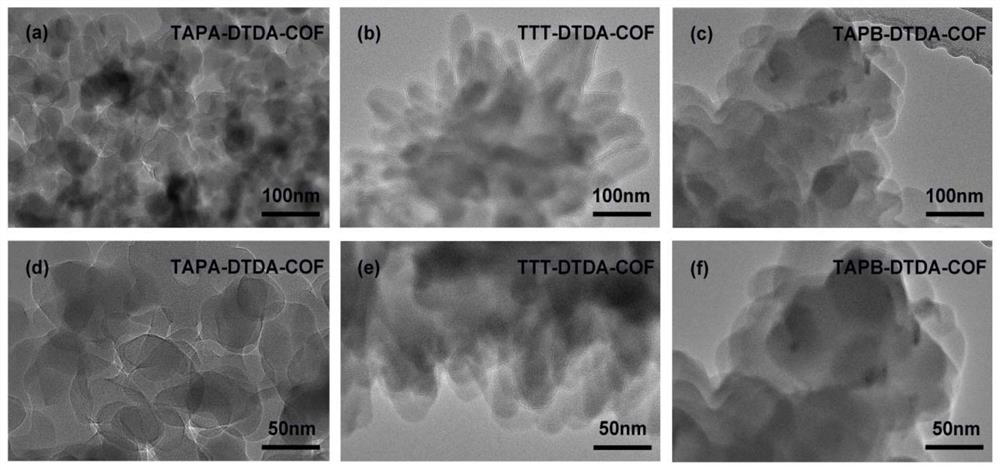
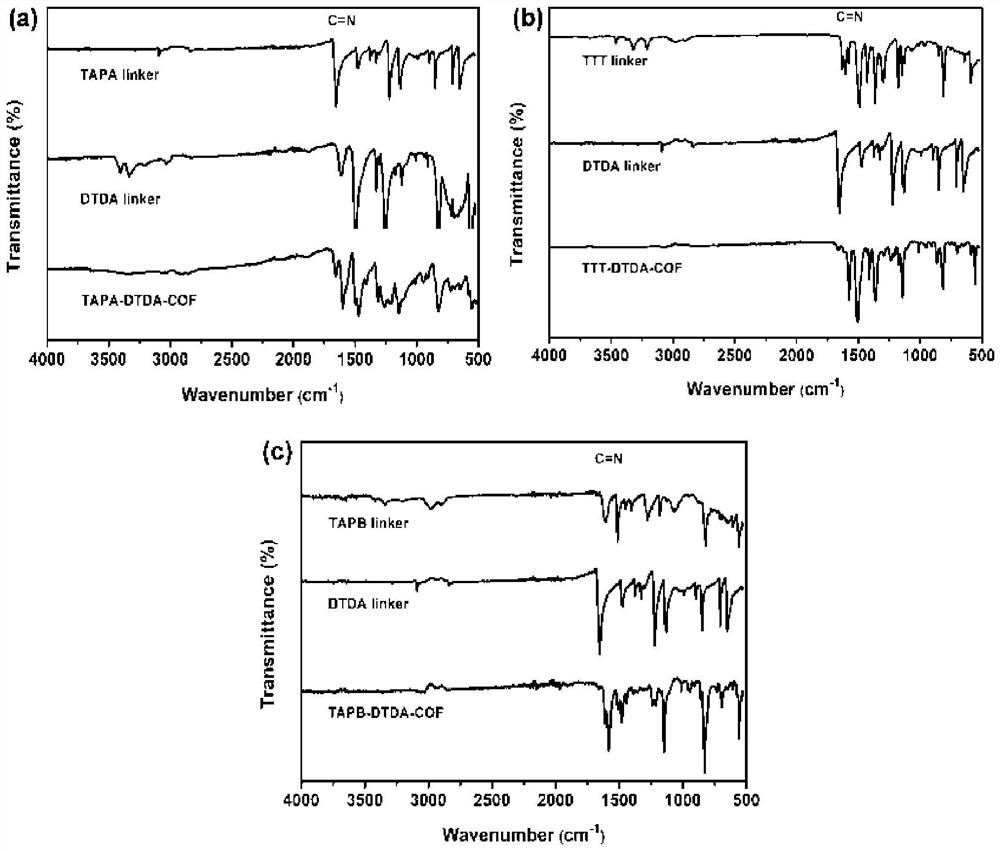
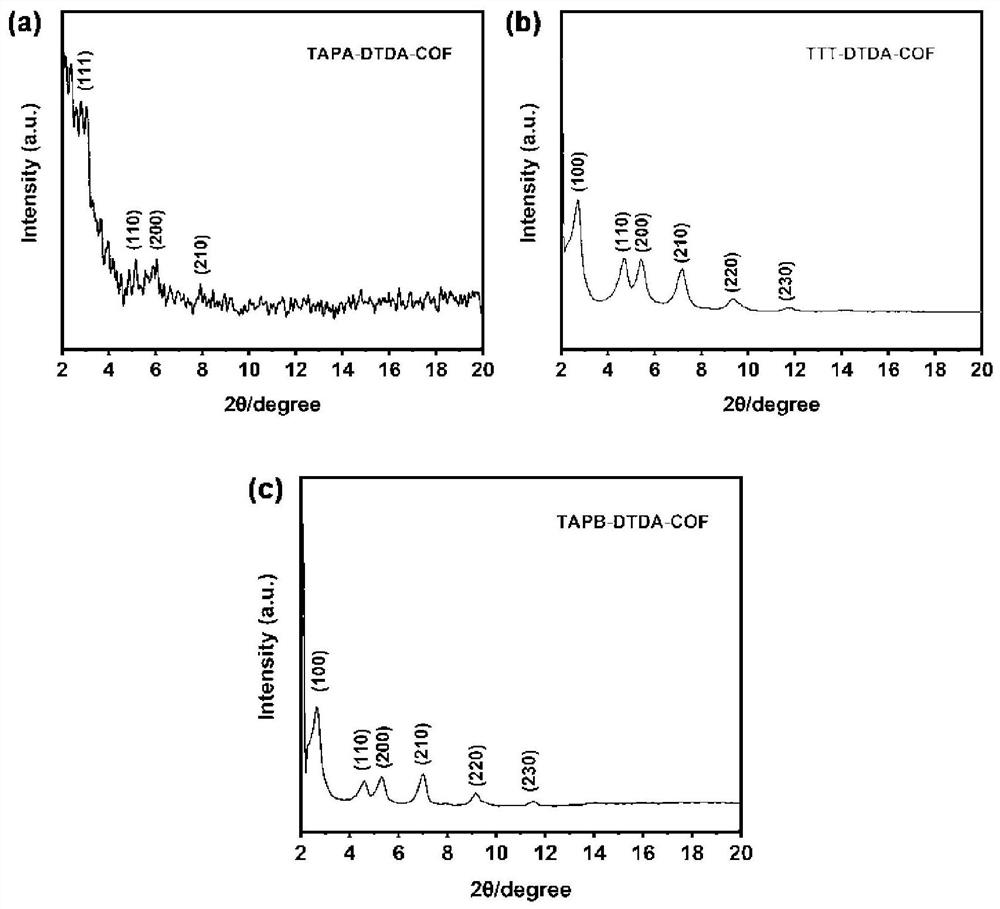
[0053] A method for preparing a COFs material with tris(4-aminophenyl)amine (TAPA) as the central building block, the specific steps are as follows:
[0054] (1) Weigh 7.30mg (0.04mmol) of DTDA and 8.80mg (0.03mmol) of TAPA into a 5mL glass tube, then add 500μL of n-butanol and 500μL of 1,2-dichlorobenzene into the above glass bottle, and ultrasonically After 30 minutes to obtain a uniform mixed solution, take 100 μL of 6 mol / L acetic acid solution and add it to the mixed solution and sonicate for 10 minutes to obtain the final solution.
[0055] (2) Transfer the uniformly mixed solution in the glass bottle to the Parker tube, freeze it quickly with liquid nitrogen, evacuate the internal pressure to a vacuum, and then seal it. After returning to room temperature and thawing, vacuumize repeatedly three times. Put the vacuumized solution in an oven at 120°C for three days of reaction, and cool to room temperature after the reaction. The product obtained by the reaction was was...
Embodiment 2
[0057] A method for preparing a COFs material with three (4-aminophenyl) benzene (TAPB) as a central building unit, the specific steps are as follows:
[0058] (1) Weigh 7.30mg (0.04mmol) of DTDA and 10.50mg (0.03mmol) of TAPA into a 5mL glass tube, then add 500μL of n-butanol and 500μL of 1,2-dichlorobenzene into the above glass bottle, and ultrasonically After 30 minutes to obtain a uniform mixed solution, take 100 μL of 6 mol / L acetic acid solution and add it to the mixed solution and sonicate for 10 minutes to obtain the final solution.
[0059] (2) Transfer the uniformly mixed solution in the glass bottle to the Parker tube, freeze it quickly with liquid nitrogen, evacuate the internal pressure to a vacuum, and then seal it. After returning to room temperature and thawing, vacuumize repeatedly three times. Put the vacuumized solution in an oven at 120°C for three days of reaction, and cool to room temperature after the reaction. The product obtained by the reaction was ...
Embodiment 3
[0061] A method for preparing a COFs material with 4,4',4"-(1,3,5-triazine-2,4,6-triyl)triphenylamine (TTT) as a central building unit, the specific steps are as follows:
[0062] (1) Weigh 7.30mg (0.04mmol) of DTDA and 10.50mg (0.03mmol) of TTT into a 5mL glass tube, then add 500μL of n-butanol and 500μL of 1,2-dichlorobenzene into the above glass bottle, and ultrasonically After 30 minutes to obtain a uniform mixed solution, take 100 μL of 6 mol / L acetic acid solution and add it to the mixed solution and sonicate for 10 minutes to obtain the final solution.
[0063] (2) Transfer the uniformly mixed solution in the glass bottle to the Parker tube, freeze it quickly with liquid nitrogen, evacuate the internal pressure to a vacuum, and then seal it. After returning to room temperature and thawing, vacuumize repeatedly three times. Put the vacuumized solution in an oven at 120°C for three days of reaction, and cool to room temperature after the reaction. The product obtained b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com