Green method for catalyzing dithioacetal/ketone to be deprotected into carbonyl compound
A technology for catalyzing dithioacetal and carbonyl compounds, which is applied in the field of green synthesis of catalyzing the deprotection of dithioacetal/ketone into carbonyl compounds, and can solve the problem of limiting the application of thioacetal/ketone protecting groups and poor functional group compatibility , reagents are expensive and other problems, to achieve good application prospects, good tolerance, easy to obtain the effect of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
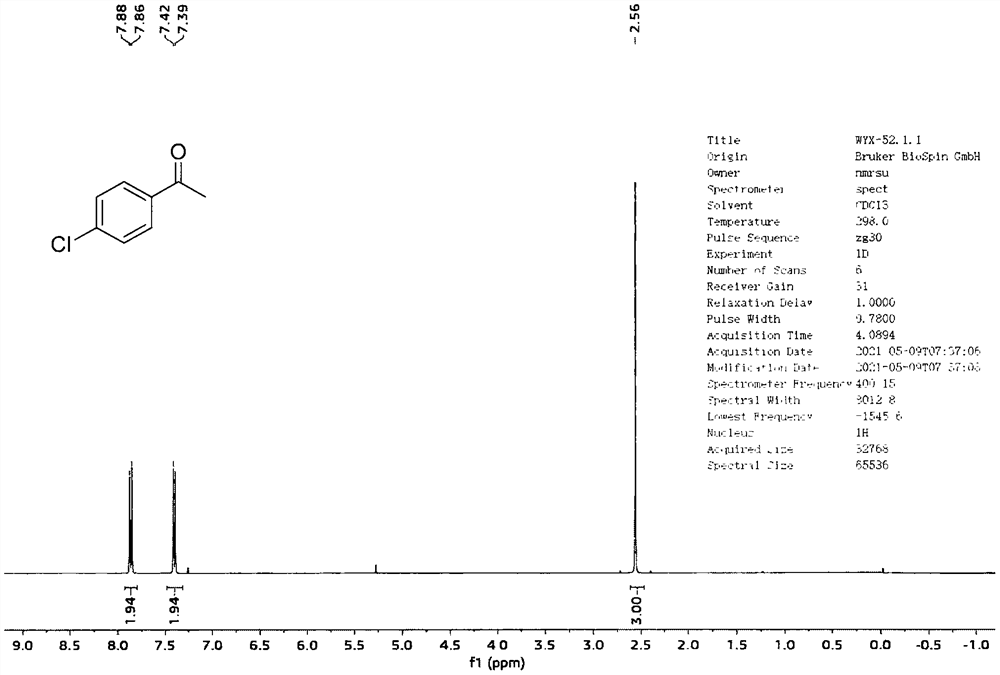
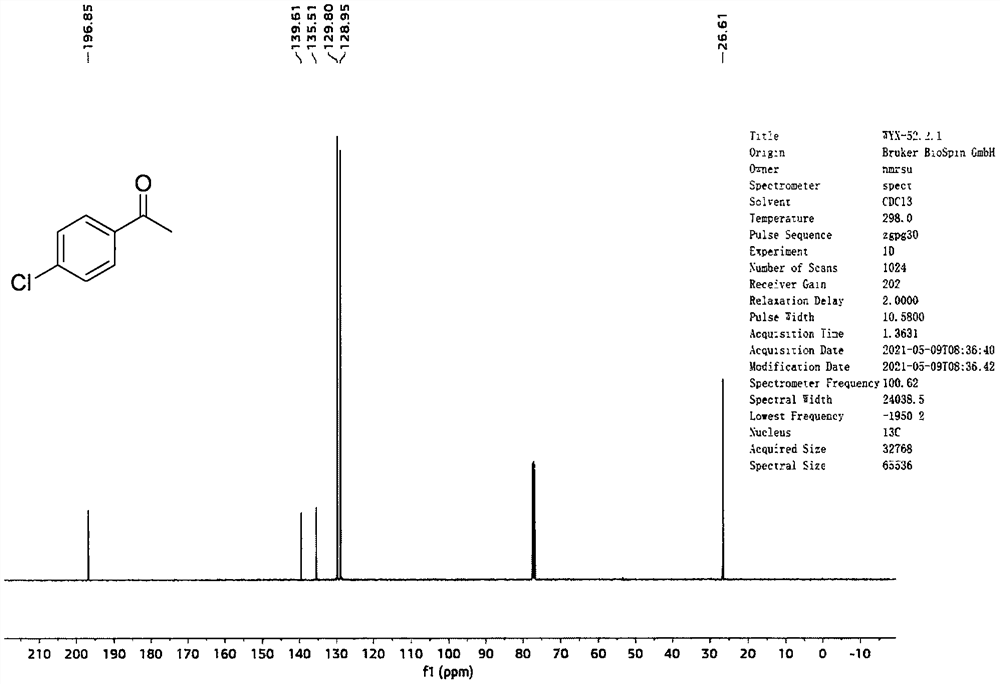
[0029] Add 40mL of ethanol and 1a (4mmol, 0.92g) into a 100mL round bottom flask in sequence, stir well, then add CeBr to the mixture of the two in sequence 3 (0.4mmol, 0.152g), H 2 o 2 Aqueous solution (30wt%, 8mmol, 0.82mL), stirred at room temperature for 10min. After the reaction is complete, use Na 2 S 2 o 3 solution (0.1M, 20 mL) to quench the reaction and extract with ethyl acetate (100 mL). The organic phase was collected and the aqueous phase was extracted with ethyl acetate (2 x 50 mL). After combining the organic phases, they were successively washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain the target product 1b (yield: 99%). The characterization data of this compound are as follows: 1 H-NMR (400MHz, Chloroform-d) δ7.93-7.80(m, 2H), 7.48-7.32(m, 2H), 2.56(s, 3H). 13 C-NMR (100MHz, Chloroform-d) δ196.8, 139.6, 135.5, 129.8, 128.9, 26.6. cm -1 ; HRMS (ESI + )(m / z)calcd.for C 8 ...
Embodiment 2
[0031]
[0032] Add 40mL of ethanol and 2a (4mmol, 1.1g) into a 100mL round bottom flask in turn, stir well, and then add CeBr to the mixture of the two in turn 3 (0.4mmol, 0.152g), H 2 o 2 Aqueous solution (30wt%, 8mmol, 0.82mL), stirred at room temperature for 10min. After the reaction is complete, use Na 2 S 2 o 3 solution (0.1M, 20 mL) to quench the reaction and extract with ethyl acetate (100 mL). The organic phase was collected and the aqueous phase was extracted with ethyl acetate (2 x 50 mL). After combining the organic phases, they were successively washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain the target product 2b (yield: 90%). The characterization data of this compound are as follows: 1H-NMR (400MHz, Chloroform-d) δ7.89-7.71(m, 2H), 7.64-7.53(m, 2H), 2.57(s, 3H). 13 C-NMR (100MHz, Chloroform-d) δ197.1, 135.9, 132.0, 129.9, 128.4, 26.6. -1 ; HRMS (ESI + )(m / z)calcd.for C 8 h 8 BrO[M...
Embodiment 3
[0034]
[0035] Add 30mL of ethanol and 3a (3mmol, 0.97g) into a 100mL round bottom flask in turn, stir well, and then add CeBr to the mixture of the two in turn 3 (0.3mmol, 0.114g), H 2 o 2 Aqueous solution (30wt%, 6mmol, 0.61mL), stirred at room temperature for 10min. After the reaction is complete, use Na 2 S 2 o 3 solution (0.1 M, 15 mL) to quench the reaction and extract with ethyl acetate (100 mL). The organic phase was collected and the aqueous phase was extracted with ethyl acetate (2 x 50 mL). After combining the organic phases, they were successively washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain the target product 3b (yield: 92%). The characterization data of this compound are as follows: 1 H-NMR (400MHz, Chloroform-d) δ7.88-7.76(m, 2H), 7.71-7.60(m, 2H), 2.56(s, 3H). 13 C-NMR (100MHz, Chloroform-d) δ197.4, 138.0, 136.5, 129.8, 101.2, 26.6. -1 ; HRMS (ESI + )(m / z)calcd.for C 8 h 8 IO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com