Medicine release-controlled microcapsule with dual-layer membrane and its preparing process
A drug controlled release and microcapsule technology, which is applied in microcapsules, capsule delivery, pharmaceutical formulations, etc., can solve the problems of inconsistent microcapsule film strength and controlled release performance, and achieve the advantages of intestinal drug delivery, rich content, Effect of good bioadhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 bilayer film structure ACA microcapsule
[0027] Under the condition of 20°C, according to the volume ratio of calcium alginate and chitosan solution of 1:2, the film-forming reaction was carried out for 25 minutes, and then the surface of the beads was covered with 2.5 volumes of sodium alginate solution with a concentration of 0.2%, and then combined with chitosan The sugar solution was subjected to the second film-forming reaction for 20 minutes. Chitosan solution concentration: 0.2%, chitosan molecular weight: 70,000 for the first time, 200,000 for the second time, and deacetylation degree: 85%. ACA microcapsules with double-layer membrane structure ( figure 1 ).
Embodiment 2
[0028] Embodiment 2 application test (1)
[0029] The double-layer membrane structure microcapsules prepared in Example 1 were not damaged during the release process of the simulated gastrointestinal fluid. The microcapsules were administered to mice, and no capsules were found in the small intestine of the mice for 48 hours.
Embodiment 3
[0030] Embodiment 3 application test (II)
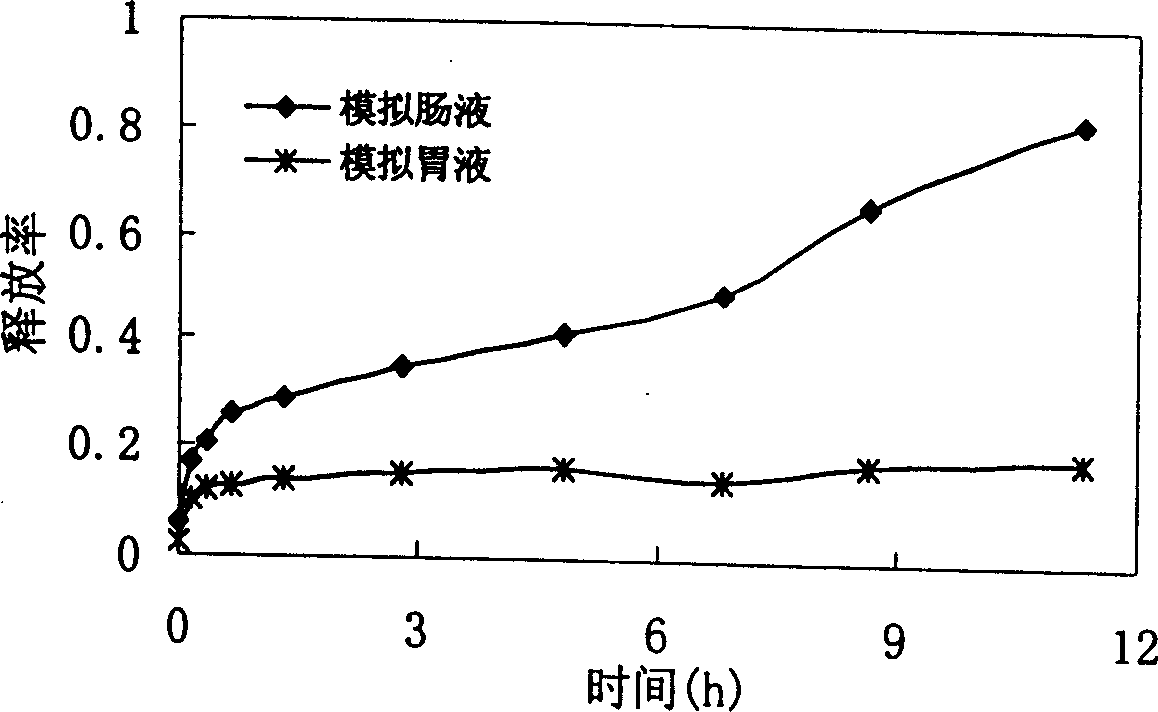
[0031] After the microencapsulated bovine hemoglobin prepared by the method of Example 1 was released in simulated gastrointestinal fluid for 7 hours, there was still a large amount of unreleased protein in the microcapsules ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com