Injectable homogeneous gels comprising multiple forms of hyaluronic acid and methods for manufacturing thereof
A technology of hyaluronic acid and cross-linked hyaluronic acid, used in medical and pharmaceutical applications, can solve problems such as poor in vivo stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 - A three-component gel composed of 89.55% CL-gel-1, 9.95% NCL-gel-1 and 0.5% DHCL-1 glue
[0056] Step 1: Preparation of hyaluronic acid-based DHCL-1. Add 6.54 g of sodium hyaluronate (HA) with a molecular weight of 1.3-2.0 MDa (pharmaceutical grade) to 54.12 g of water and 1.96 g of 1,4-butanediol diglycidyl ether (BDDE), and mix the mixture manually, It was then homogenized by a Thinky planetary mixer at 2000 rpm. Thereafter, 11.23 g of 1M sodium hydroxide (NaOH) solution was added to the mixture, bringing it to a total of 73.85 g. The mixture was then homogenized at 300 rpm for 120 minutes. The mixture was then placed in an oven set at 45°C for 3 hours and then in a 25°C oven for an additional 15 hours. The mixture was ground and then 70.59 g of the gel was neutralized by adding phosphate buffer at a pH of about 7.3 and adjusting to a final pH with 1 N HCl solution (103.29 g of neutralizing solution in total) to give a pH of about 7. Final pH. The...
Embodiment 2
[0065] Example 2 - Three components consisting of 49.75% CL-gel-1, 49.75% NCL-gel-1 and 0.5% DHCL-1 gel
[0066] DHCL-1, CH-Gel 1 and NCL-Gel 1 were prepared as described in Example 1.
[0067] Step 4: Prepare the final body.
[0068] 100 g of CL-gel-1 was added to 100 g of NCL-gel-1. 1 g of DHCL-1 was added to the mixture and the mass was manually mixed and then homogenized at 2000 rpm. Finally the gel was degassed in vacuo by subjecting it to vacuum for 30 min, then milled and filled into 1.25 mL glass syringes which were sterilized by steam autoclave at 121°C for 20 min. minute. Viscous and viscoelastic gels are formed.
[0069] The pH of the final gel was about 7. The gel is easily injectable through a needle: 20 to 40 N of force is required to push the gel through a 25G / 16mm PIC needle at a push rate of 1 mL / min.
Embodiment 3
[0070] Example 3 - Three-component gel consisting of 89.1% CL-gel-1, 9.9% NCL-gel-1 and 1% DHCL-1
[0071] DHCL-1, CH-Gel 1 and NCL-Gel 1 were prepared as described in Example 1.
[0072] Step 4: Prepare the final body.
[0073] 13.50 g CL-gel-1 was added to 1.50 g NCL-gel-1. 0.15g DHCL-1 was added to the mixture and the mass was mixed manually and then homogenized at 2000rpm. Finally the gel was degassed in vacuo by subjecting it to vacuum for 30 min, then milled and filled into 1.25 mL glass syringes which were sterilized by steam autoclave at 121°C for 20 min. minute. Viscous and viscoelastic gels are formed.
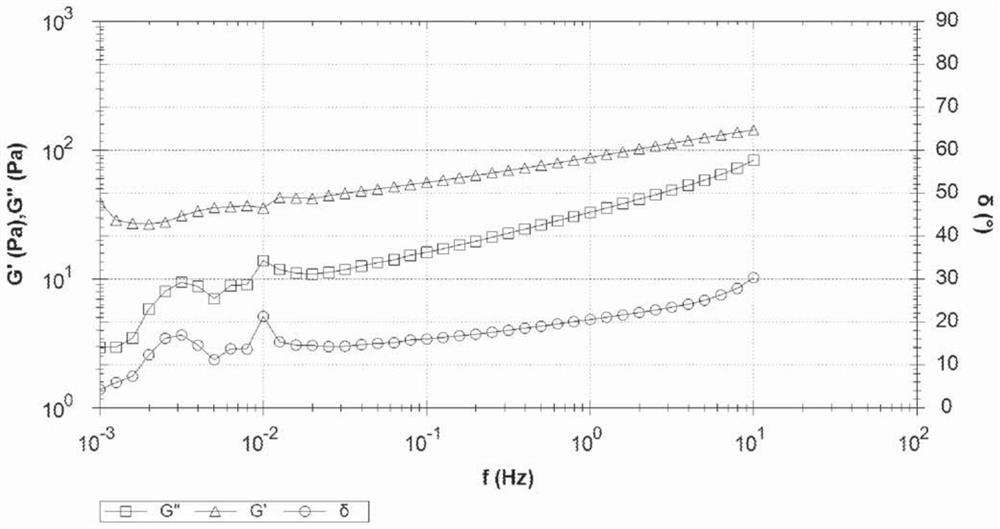
[0074] The pH of the final gel was about 7. The gel is easily injectable through a needle: an injection force of 34N is required to push the gel through a 25G / 16mm PIC needle at a push rate of 1 mL / min. The viscosity of the gel was 197 Pa*s and G' and G" measured at 0.5% shear strain at 1 Hz were 125 Pa and 46 Pa, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com