N-heterocyclic carbazole compound and application thereof
A compound and carbazole technology, applied in the field of deep blue light materials, to achieve the effects of simple operation, mild synthesis conditions and excellent film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
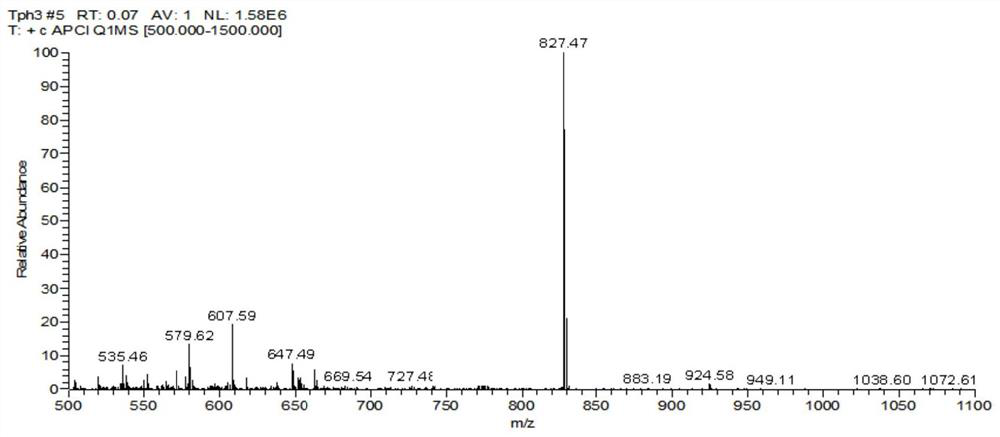
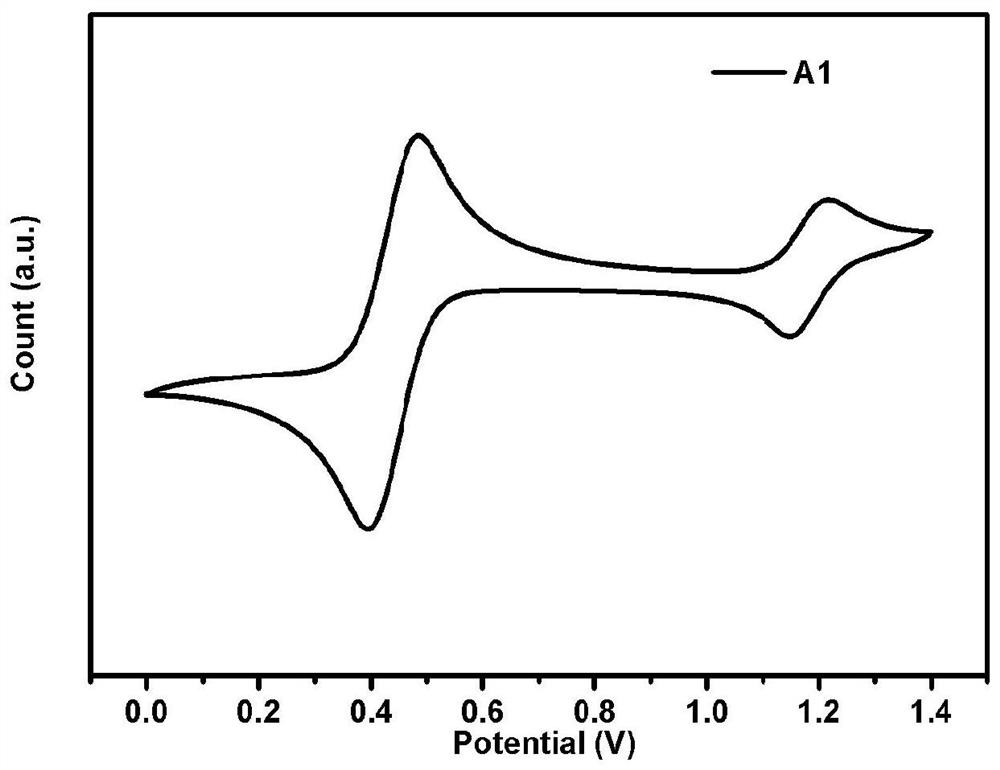
[0092] Embodiment 1 Preparation of a nitrogen heterocyclic carbazole compound (A1)
[0093] S1. Mix 3,6-di-tert-butyl-9H-carbazole (1.12g, 4.0mmol), 1-bromo-4-iodobenzene, cuprous iodide, and potassium carbonate at a molar ratio of 1:1.2:0.2:2 Add 20mL of anhydrous N,N-dimethylformamide to a two-necked flask, vacuumize the flask and replace it in dry nitrogen three times, heat and reflux under nitrogen at 140°C and stir for 12h, then cool and filter , extracted with ethyl acetate, the organic layer was separated and dried with anhydrous magnesium sulfate, filtered and evaporated, the crude product was subjected to column chromatography (petroleum ether as eluent) to obtain 1.15 g of white powder (intermediate M1, yield was 66%).
[0094] The reaction formula is as follows:
[0095]
[0096] S2. The intermediate M1 (521 mg, 1.20 mmol), 2,4-biphenyl-6-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaboron) obtained in step S1 Alkyl-2-yl)phenyl)-1,3,5-triazine, tetrakis(triphenylphosphin...
Embodiment 2
[0098] Embodiment 2 Preparation of a nitrogen heterocyclic carbazole compound (A2)
[0099]S1. Mix 3,6-di-tert-butyl-9H-carbazole (279mg, 1.2mmol), methyl 5-bromo-2-iodobenzoate, cuprous iodide, and potassium carbonate at a ratio of 1:1.2:0.2:2 The molar ratio was added to a two-neck flask containing 5 mL of anhydrous N,N-dimethylformamide, the flask was evacuated and replaced in dry argon three times, and heated under reflux at 140°C under argon and stirred for 12 hours. , cooled, filtered, extracted with ethyl acetate, the organic layer was separated and dried with anhydrous magnesium sulfate, filtered and evaporated, the crude product was subjected to column chromatography (petroleum ether as eluent) to obtain 365 mg of white powder (intermediate M2 , the yield was 74%). The reaction formula is as follows:
[0100]
[0101] S2. Add phenylmagnesium bromide into the flask under argon and stir, then slowly add 1 mL of tetrahydrofuran dissolved in intermediate M2 (365 mg, ...
Embodiment 3
[0105] Embodiment 3 Preparation of a nitrogen heterocyclic carbazole compound (A1)
[0106] S1. Mix 3,6-di-tert-butyl-9H-carbazole (1.12g, 4.0mmol), 1-bromo-4-iodobenzene, cuprous iodide, and potassium carbonate at a molar ratio of 1:1.2:0.3:3 Pour into a two-necked flask filled with 20 mL of anhydrous dimethyl sulfoxide, vacuumize the flask and replace it in dry argon three times, heat and reflux under argon at 120°C for 15 h, cool, filter, and use Extracted with ethyl acetate, the organic layer was separated and dried with anhydrous magnesium sulfate, filtered and evaporated, the crude product was subjected to column chromatography (eluent was petroleum ether) to obtain 1.06g white powder (intermediate M1, yield: 61 %). Reaction formula is with embodiment 1.
[0107] S2. The intermediate M1 (521 mg, 1.20 mmol), 2,4-biphenyl-6-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaboron) obtained in step S1 Alkyl-2-yl)phenyl)-1,3,5-triazine, tetrakis(triphenylphosphine)palladium, and potassiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com