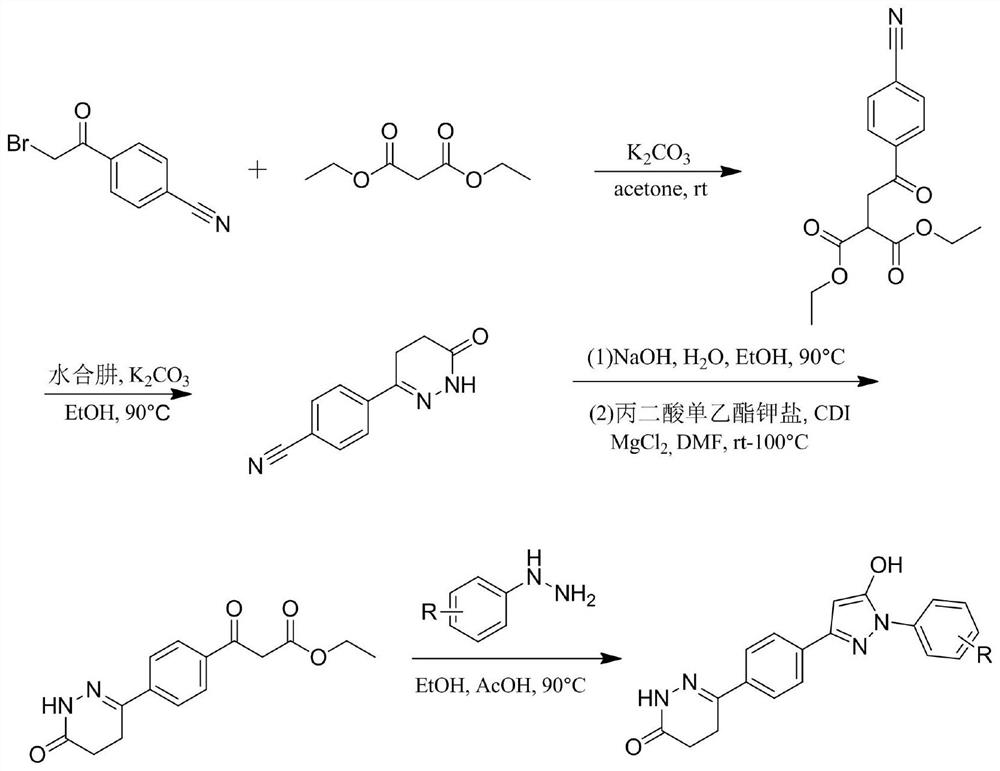
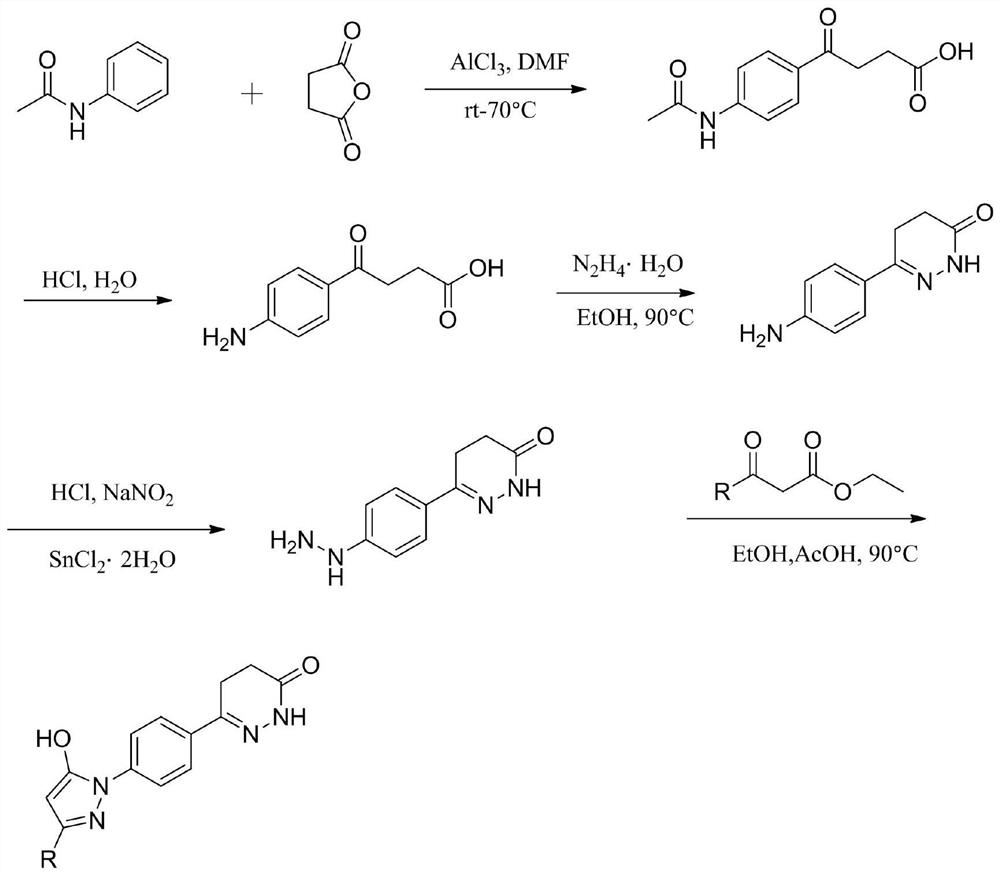
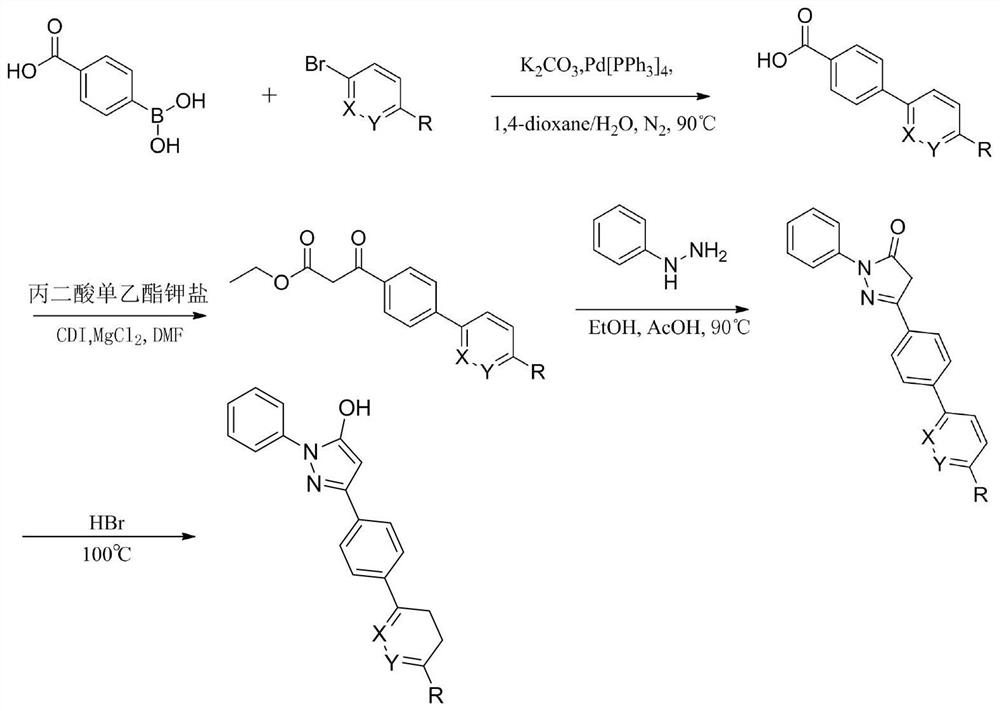
Pyrazol alcohol-pyridazinone coupling compound and pharmaceutical composition thereof and application of pyrazol pyridazinone-pyridazinone coupling compound in medicine
A technology of pyridazinone and pyrazolol, which is applied in the field of medicinal chemistry, can solve the problems of increased drug burden for patients, and achieve the effects of reduced drug burden, excellent neuroprotective effect, and low drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] This embodiment provides a compound of formula 1- particular compound of Formula 7 and Table 1:
[0033] Table 1
[0034]
[0035] The compound 6- (4- (5-hydroxy-1-phenyl--1H- pyrazol-3-yl) phenyl) -4,5-dihydro-pyridazin -3 (2H) - one; Mp257- 261 ℃. 1 H NMR (400MHz, DMSO-D 6 ) Δ11.88 (s, 1H), 10.95 (s, 1H), 7.88 (d, J = 8.5Hz, 2H), 7.81 (dd, J = 10.4,8.4Hz, 4H), 7.49 (dd, J = 10.8 , 5.1Hz, 2H), 7.30 (t, J = 7.4Hz, 1H), 6.08 (s, 1H), 2.98 (t, J = 8.2Hz, 2H), 2.47 (t, J = 8.2Hz, 2H). 13 C NMR (100MHz, DMSO-D 6 ) Δ167.49,154.33,149.56,149.39,139.26,135.65,134.58,129.40,126.34,126.28,125.52,121.68,85.77,26.47,22.26.HRMS (ESI) calcd for C 19 Hide 17 N 4 O 2 [M + h] + : 333.1352, found 333.1341.
[0036] Compound 2 6- (4- (5-hydroxy-1- (pyridin-2-yl) lH-pyrazol-3-yl) phenyl) -4,5-dihydro-pyridazin -3 (2H) - ketones; Mp253-256 ℃. 1 H NMR (600MHz, DMSO-D 6 ) Δ12.60 (s, 1H), 10.99 (s, 1H), 8.49 (dd, J = 4.9,0.9Hz, 1H), 8.10 (t, J = 7.4Hz, 1H), 7.93 (t, J = 8.6 hz, 3H), 7.83 (d, J ...
Embodiment 2
[0062] This embodiment provides a compound 8-16, which is as follows:
[0063]
[0064] The specific chemical structural formula of Compound 8-16 is shown in Table 3:
[0065] table 3
[0066]
[0067]
[0068] Compound 8 is 6- (4- (5-hydroxy-3-methyl-1H-pyrazole-1-yl) phenyl) -4,5-dihydroxazine-3 (2H)-ketone; MP258- 261 ° C. 1 H NMR (600MHz, DMSO-D 6 Δ11.69 (S, 1H), 10.94 (S, 1H), 7.81 (S, 4H), 5.41 (S, 1H), 2.96 (T, J = 8.2 Hz, 2H), 2.46 (T, J = 8.2 Hz, 2H), 2.12 (D, J = 5.7 Hz, 3H). 13 C NMR (150MHz, DMSO-D 6 Δ167.46, 153.92, 149.31, 140.13, 132.63, 126.74, 12.20, 117.79, 88.35, 24.45, 22.21, 14.78.HRMS (ESI) Calcd for C 14 Hide 15 N 4 O 2 [M + h] + : 271.1195, Found271.1200.
[0069] Compound 9 is 6- (4- (5-hydroxy-3-phenyl-1H-pyrazole-1-yl) phenyl) -4,5-dihydroxazine-3 (2H)-ketone; MP254- 257 ° C. 1 H NMR (600MHz, DMSO-D 6 δ12.06 (S, 1H), 10.98 (S, 1H), 7.93 (D, J = 8.7 Hz, 2H), 7.86 (DD, J = 11.6, 8.5 Hz, 4H), 7.43 (T, J = 7.5 Hz, 2H), 7.34 (T, J = 7.2 Hz, 1H), 6.05 (S...
Embodiment 3
[0096] The present embodiments provide compounds 17-19, having the general formula:
[0097]
[0098] Specific compounds of formula 17-19 as shown in Table 5:
[0099] table 5
[0100]
[0101] Compound 17 3- (4- (6-methoxy-pyridazin-3-yl) phenyl) -1-phenyl -1H- pyrazole -5 (4H) - one; M.p.198-201 ℃. 1 H NMR (600MHz, DMSO-D 6 ) Δ11.92 (s, 1H), 8.22 (d, J = 9.3Hz, 1H), 8.14 (d, J = 8.3Hz, 2H), 7.99 (d, J = 8.4Hz, 2H), 7.85 (d, J = 8.0Hz, 2H), 7.50 (t, J = 7.9Hz, 2H), 7.32 (dd, J = 17.3,8.3Hz, 2H), 6.13 (s, 1H), 4.09 (s, 3H). 13 C NMR (150MHz, DMSO-D 6 ) Δ164.58,154.87,154.35,149.40,139.29,135.59,134.83,129.41,128.15,126.97,126.29,125.98,121.70,118.06,85.83,54.97.HRMS (ESI) calcd for C 20 Hide 17 N 4 O 2 [M + h] + : 345.1352, found345.1351.
[0102] Compound 18 3- (4- (6-bromo-pyridazin-3-yl) phenyl) -1-phenyl -1H- pyrazole -5 (4H) - one; M.p.243-246 ℃. 1 H NMR (400MHz, DMSO-D 6 ) Δ11.91 (s, 1H), 8.27 (d, J = 9.1Hz, 1H), 8.21 (d, J = 8.3Hz, 2H), 8.12 (d, J = 9.0Hz, 1H), 8.03 (d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com