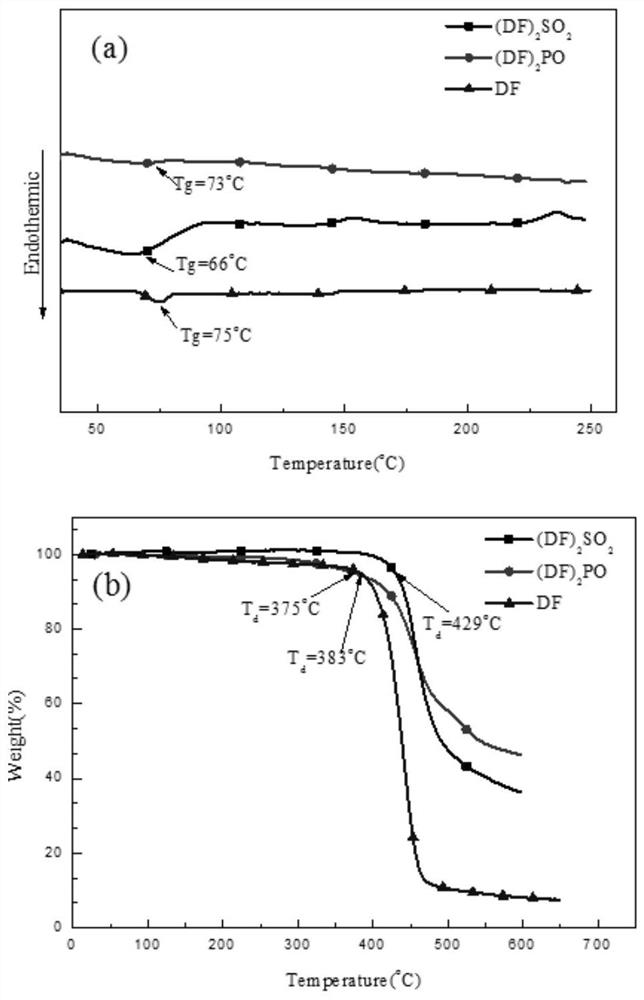
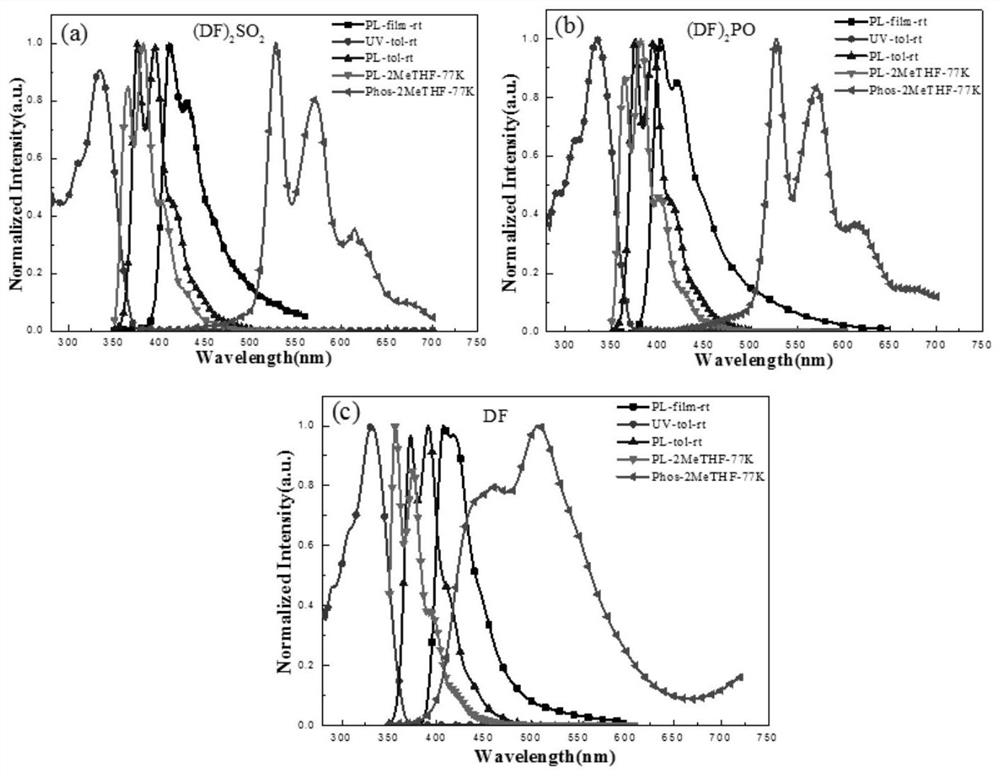
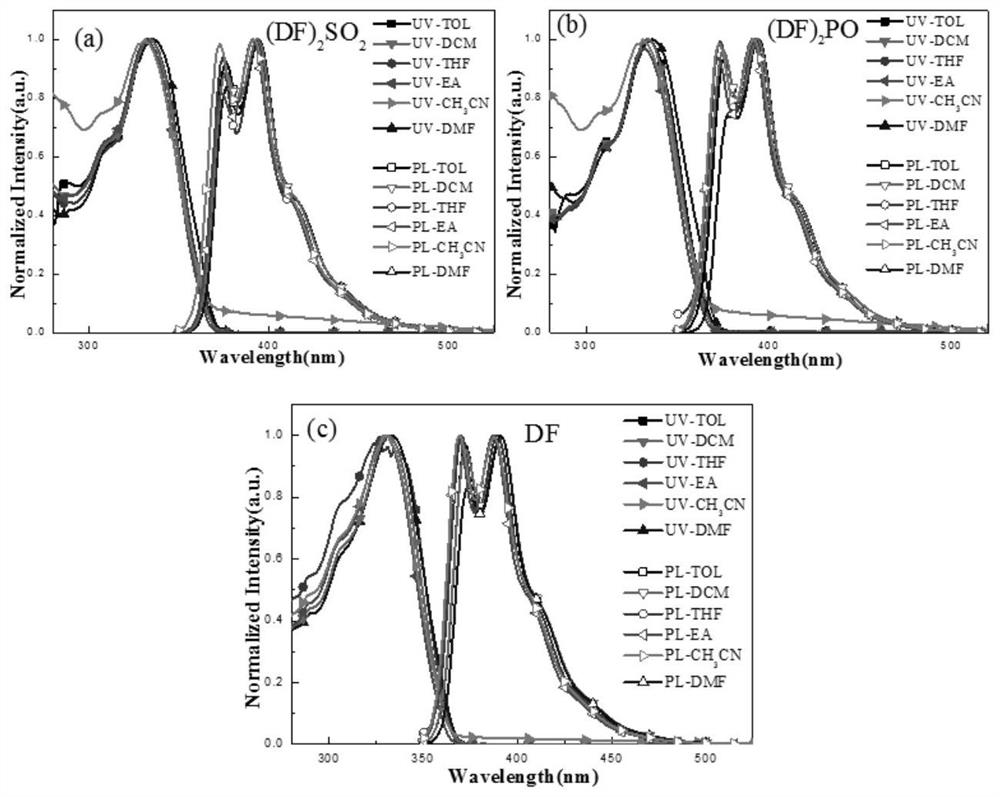
Blue luminescent materials based on bifluorene derivative
A technology of blue light-emitting materials and fluorene derivatives, applied in the directions of light-emitting materials, compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, etc., to improve thermal stability, reduce interactions, and reduce emission. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: The synthesis route of the electron donor bisfluorene DF is as follows
[0028]
[0029] The specific synthesis process is as follows:
[0030] Take a two-necked flask, add 4.27g of p-bromophenol, 6.63g of anhydrous potassium carbonate, 33mL of N,N-dimethylformamide (DMF), 3.3g of bromobutane, and react at 80°C for 10 hours to obtain 1-bromo - 4-butoxybenzene 3.6 g (66% yield).
[0031] Take a two-necked flask, prepare 3.62 g of 1-bromo-4-butoxybenzene into Grignard reagent, add 3.1 g of 2-bromofluorenone, 60 mL of tetrahydrofuran, and reflux at 65°C for 20 hours to obtain 2-bromo-9-(4 -butoxyphenyl)-9-fluorenol 4.4 g (55% yield).
[0032] Take a two-neck flask, add 1.8g of 2-bromofluorenone, 6.6g of phenol, and 10mL of methanesulfonic acid, and react at 50°C for about 20h to obtain 1.87g of a white powdery product.
[0033] Take a two-necked flask, add 1.7g of the white powder obtained in the previous step, 0.62g of anhydrous potassium carbonate, 30mL ...
Embodiment 2
[0038] Embodiment 2: compound (DF) 2 SO 2 and (DF) 2 The synthetic route of PO is as follows
[0039]
[0040] (DF) 2 SO 2 Preparation: Take the reaction test tube, add DF 1.5g (1.94mmol), 4,4-dibromodiphenyl sulfone 0.2g, potassium tert-butoxide 0.175g, tert-butyldiphenylphosphine 0.04g, tri-(diphenyl Benzylacetone) dipalladium 0.1g, toluene 4mL, react at 110°C for 30h to obtain the product (DF) 2 SO 2 0.27g.
[0041] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.77(dd,J=13.2,7.3Hz,12H),7.57(d,J=9.9Hz,4H),7.53–7.47(m,4H),7.38(q,J=8.0Hz,10H ),7.34–7.28(m,4H),7.27(d,J=5.6Hz,1H),7.25–7.23(m,1H),7.18–7.13(m,8H),7.07(d,J=8.7Hz, 4H),6.77(t,J=7.0Hz,12H),3.90(t,J=5.9Hz,12H),1.78–1.68(m,12H),1.50–1.42(m,12H),0.95(td,J =7.3, 2.6Hz, 18H). 13 CNMR (101MHz, CDCl 3 ,ppm)δ158.23,157.94,152.62,152.33,152.26,141.28,140.39,139.70,139.47,139.40,139.10,137.75,129.15,129.10,128.95,127.93,127.75,127.34,126.53,126.06,124.61,124.51,114.39,114.10 ,67.58,64.84,64.29,31.36,19.25,13.84. MALDI-TOF, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com