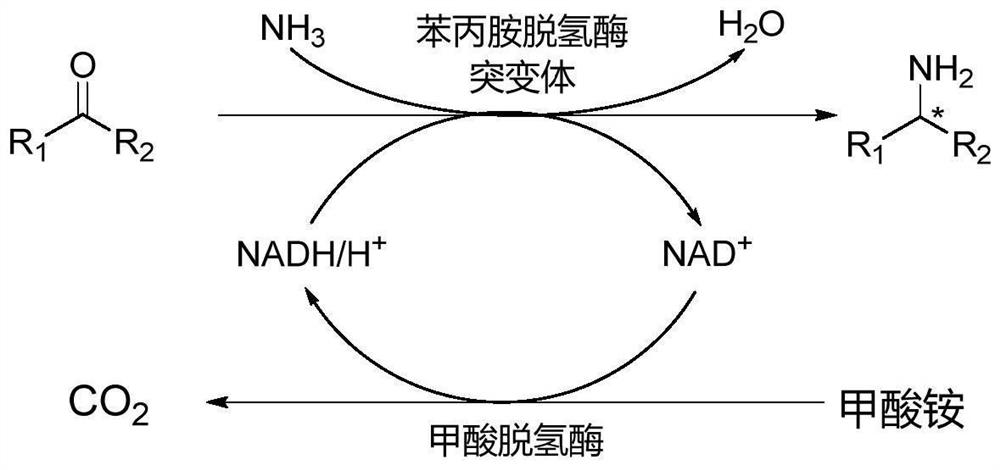
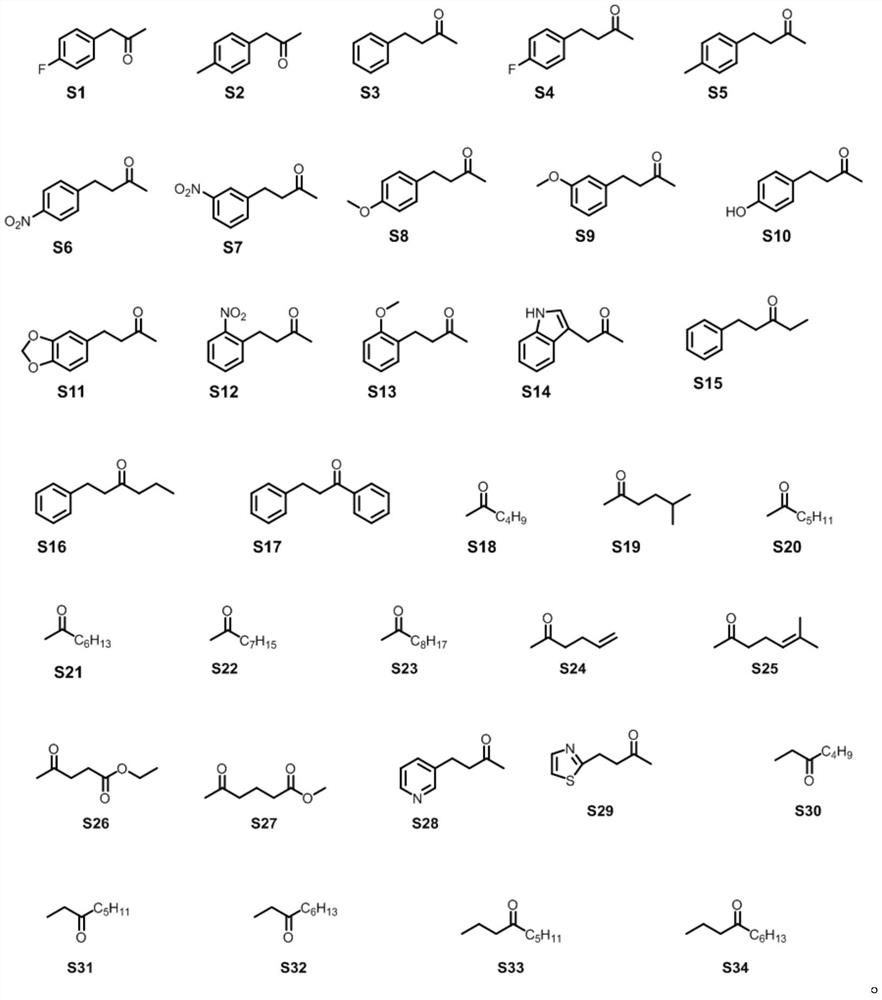
Amphetamine dehydrogenase mutant and application thereof in chiral amine synthesis
An amine dehydrogenase and mutant technology, applied in the field of bioengineering, can solve the problems of narrow application range, inability to catalyze large sterically hindered carbonyl substrates, etc., and achieve high catalytic activity, high substrate concentration tolerance, and product optical high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The construction of embodiment 1 amphetamine dehydrogenase GkAmDH mutant
[0055] Using the recombinant plasmid GkAmDH as the parent, design primers for the sites to be mutated, and the sequences of the upstream and downstream primers are shown in the table.
[0056] The gene sequence of amphetamine dehydrogenase GkAmDH is shown in SEQ ID No.1.
[0057] The primer purification method is PAGE, and the primer structure is: upstream primer: 15-20 bases before the mutation site + mutation base + 15-20 bases after the mutation site; downstream primer: reverse complementary sequence of the entire upstream primer ;Primers should be stored at -20°C for long-term storage. The PCR system contained 10 μL PrimeSTAR HS, 1 μL of upstream and downstream primers (10 ng / μL), 1 μL of recombinant plasmid (100 ng / μl), 0.5 μL DMSO and 6.5 μL ddH2O. The PCR amplification program was as follows: pre-denaturation at 98°C for 10 seconds, followed by 20 cycles of denaturation at 98°C for 10 se...
Embodiment 2
[0069] Example 2 Construction of amphetamine dehydrogenase GkAmDH mutant recombinant expression transformant
[0070] Transform the recombinant plasmid ligation solution containing the target fragment of the amphetamine dehydrogenase mutant obtained by PCR amplification in Example 1 into E.coli BL21, and pick positive clones to obtain a series of recombinant expression transformants E.coli BL21(DE3) / pET28a-GkAmDH M3~8, the correctness of the target gene was confirmed by gene sequence determination.
Embodiment 3
[0071] Embodiment 3 Preparation of amphetamine dehydrogenase GkAmDH mutant
[0072] The recombinant expression transformant obtained in Example 2 was inoculated into LB medium containing 50 μg / mL kanamycin, cultured in a shaker at 37° C. for 12 hours, and then inoculated with an inoculation amount of 1% (v / v) Put it into a shaker flask containing 100mL TB medium, put it into a shaker at 37°C and 180rpm for culture, when the OD of the culture solution 600 When it reaches about 0.6-0.8, add isopropyl-β-D-thiogalactopyranoside (IPTG) with a final concentration of 0.2mmol / L, and induce culture at 16°C for 24 hours. After the cultivation, the culture solution was centrifuged at 8000rpm to collect the cells. The collected cells were resuspended with 10 mL of Tris-HCl buffer (100 mM, pH 8.0), and subjected to the following ultrasonic treatment in an ice-water bath: 400 W power, working for 4 s, resting for 6 s, 99 cycles. The crushed solution was centrifuged at 4°C and 10,000 rpm f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com