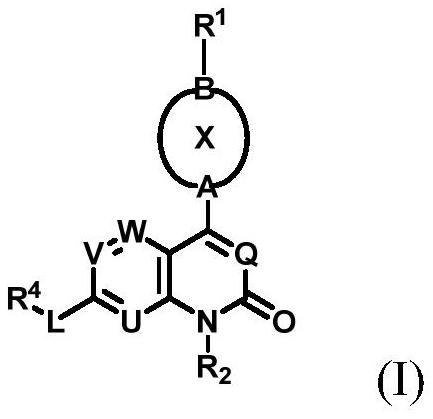
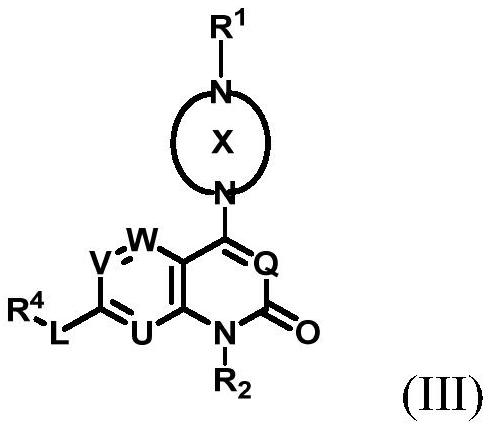
Aryl or heteroaryl pyridone or pyrimidone derivative as well as preparation method and application thereof
A technology of heteroaryl and alkyl, applied in the field of aryl or heteroaryl pyridone or pyrimidinone derivatives and their preparation, can solve the problems such as no drug approval and listing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
preparation example Construction
[0256] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of formula (I) or its crystal form, pharmaceutically acceptable salt, hydrate or solvent of the present invention compounds are mixed to form a pharmaceutical composition.
[0257] The present invention also provides a treatment method, which comprises the steps of: administering the compound of formula (I) described in the present invention, or its crystal form, pharmaceutically acceptable salt, hydrate or solvate to the subject in need of treatment, Or administer the pharmaceutical composition of the present invention for selectively inhibiting KRAS G12C .
[0258] Compared with the prior art, the present invention has the following main advantages:
[0259] (1) The compound is to KRAS G12C Has a good selective inhibitory effect;
[0260] (2) The compound has better pharmacodynamics, pharm...
Embodiment 1
[0270] Example 1 4-((S)-4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4- Preparation of methyl-2-(methylsulfonyl)pyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one
[0271]
[0272] Step 1. Preparation of 4-methyl-2-(methylsulfonyl)-3-nitropyridine
[0273] 2-Chloro-4-methyl-3-nitropyridine (3 g, 17.4 mmol) was dissolved in dimethyl sulfoxide (25 ml), and sodium methylsulfinate (2.7 g, 26.2 mmol) was added to the solution. The mixture was stirred at 120°C for 1 hour, quenched with 100 mL of water, and then extracted 3 times with 100 mL of ethyl acetate. The combined organic phases were washed 3 times with 50 mL of brine, then the organic phase was dried and concentrated. The residual solid was slurried with ethanol / methanol / ethyl acetate (40ml / 5ml / 5ml), filtered with suction and dried to obtain the title compound (2.3g, 61%).
[0274] Step 2. Preparation of 4-methyl-2-(methylsulfonyl)pyridin-3-amine
[0275] 4-Methyl-2-(methylsulfonyl)-3-nitropy...
Embodiment 2
[0293] Example 2 4-((S)-4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2- Methyl-4-(methylsulfonyl)pyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one
[0294]
[0295] LC-MS: m / z 597 (M+H) + . 1 H NMR (400MHz, CDCl 3 )δ8.93(d,J=5.2Hz,1H),
[0296]8.65(brs,1H),7.95-7.89(m,2H),7.26(m,1H),6.72-6.75(m,3H),6.45-6.39(m,1H),5.85-5.81(m,1H), 5.25-4.30(m,3H),4.20-3.50(m,3H),3.30-3.00(m,4H),2.46(d,J=10.0Hz,3H),1.51-1.48(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com