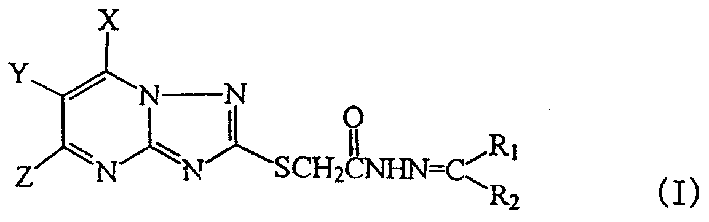
Syntehsis and activity of triazolo-pyrimido-thioacetyl hydrazone compounds
A technology of azolopyrimidine thioacetyl and azolopyrimidine, which is applied in the synthesis and application field of triazolopyrimidine thioacetylhydrazone compounds, can solve rare problems and achieve the effect of significant specific killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0021] Example 2 preparation of
[0022] Dissolve 0.5 mol of ethyl 5-amino-1,2,4-triazole-3-thioacetate and 0.5 mol of dimethyl malonate in 200 ml of absolute ethanol, then add 215 g of 25% sodium methoxide methanol solution, reflux reaction for 60 hours, cooling, suction filtration, washing with absolute ethanol twice, the solid was first dissolved in 1000ml of water, then acidified with concentrated hydrochloric acid, and a white solid was obtained by suction filtration with a yield of 82%, m.p.99~100°C .
[0023] Elemental Analysis: Calculated C% 40.03 H% 3.70 N% 20.74
[0024] Measured value C% 40.35 H% 3.23 N% 21.05
Embodiment 3
[0025] Example 3 preparation of
[0026] Add 0.5mol of 5,7-dihydroxy-1,2,4-triazolo[1,5-a]pyrimidine-2-thioacetic acid ethyl ester and 1.5mol of phosphorus oxychloride into 1200ml of acetonitrile, Reflux for 3 hours, stand overnight, filter, remove the solvent from the filtrate, then add an equal amount of ice water and dichloromethane, separate the organic phase, dry with anhydrous magnesium sulfate, concentrate until crystals are precipitated, the yield is 81%, m.p.79 ~80°C.
[0027] Elemental Analysis: Calculated C% 35.19 H% 2.61 N% 18.20
[0028] Measured value C% 35.02 H% 2.32 N% 18.55
Embodiment 4
[0029] Example 4 preparation of
[0030] Add 0.05mol of 5,7-dichloro-1,2,4-triazolo[1,5-a]pyrimidine-2-thioacetic acid ethyl ester and 60ml of methanol into the reaction flask, and add 23.5g ( 0.11mol) methanol solution containing 25% sodium methoxide, react at room temperature for 2 hours, add 100ml of water, then neutralize with 3N hydrochloric acid, and filter with suction to obtain a white solid with a yield of 84%, m.p.168-169°C.
[0031] Elemental Analysis: Calculated C% 44.30 H% 4.70 N% 18.79
[0032] Measured value C% 44.02 H% 4.33 N% 18.35
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com