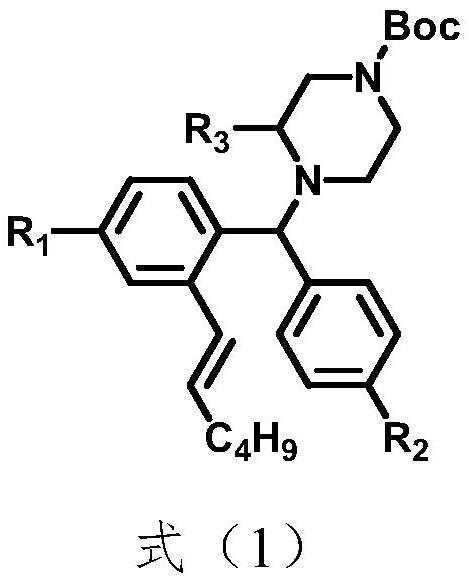
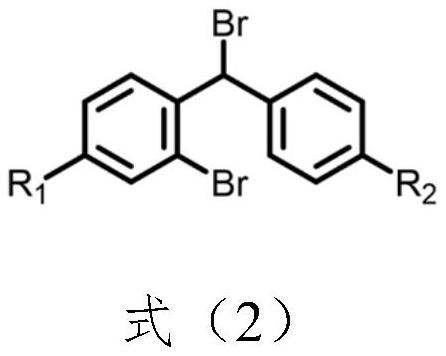
Benzhydryl piperazine compound containing straight-chain olefin and preparation method of benzhydryl piperazine compound
A technology of benzhydrylpiperazine and straight-chain olefins, which is applied in the field of medicine, can solve the problems of unreported synthesis of new benzhydrylpiperazine compounds, and achieve mild conditions, high reaction yield, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The above-mentioned preparation method of a benzhydryl piperazine compound containing linear olefins specifically comprises the following steps:
[0077] Step 1: Dissolve compound SM1 in the first solvent, add compound SM2 and alkali, and reflux the system until the reaction is complete. After the reaction is completed, the system temperature is cooled to room temperature, filtered, spin-dried, and purified to obtain compound A;
[0078] Step 2: Dissolve the compound A in the second solvent, add 1-alkenylhexylboronic acid, base, catalyst, and ligand in sequence, reflux reaction under nitrogen protection until the reaction is complete, and spin the system to dry to obtain the target compound.
[0079] In the above step 1, the first solvent is selected from at least one of acetonitrile, dichloroethane, tetrahydrofuran, and 1,2-dioxane; the base is selected from potassium carbonate, sodium carbonate, potassium acetate, At least one of sodium acetate and N,N-diisopropylethy...
Embodiment 1
[0098] Below, with R 1 selected from hydrogen; R 2 selected from hydrogen; R 3 selected from -CH 2 OAc; as an example, to illustrate the preparation method of the above-mentioned benzhydrinyl piperazine compound containing linear olefin in the embodiment of the present invention.
[0099] Step 1: Synthesis of Compound B1
[0100]
[0101] Compound SM1 (20g, 61.34mmol, 1eq) was dissolved in acetonitrile (150mL), compound SM2 (15.85g, 61.36mmol, 1eq) and potassium carbonate (17g, 123.0mmol, 2eq) were added, and the system was refluxed for 4 hours. After the reaction was completed, the system was cooled to room temperature and then filtered. After the solvent was spin-dried, silica gel column chromatography gave 27.51 g of a white solid, namely compound B1, with a yield of 87.4% and a purity of 98.1%.
[0102] The structural characterization data of compound B1 are as follows:
[0103] [M+H] + =503.16
[0104] 1 H-NMR (300MHz, CDCl 3 )δ7.79(m,1H),7.48(m,3H),7.28(m,3H)...
Embodiment 2-11 and comparative example 1-5
[0120] The steps of Examples 2-11 and Comparative Examples 1-5 are the same as the above-mentioned Example 1, only part of the process conditions of Step 2 are changed. In order to make the results more intuitive and clear, Examples 2-11 and Comparative Examples 1-5 adopt the form of a list to describe the present invention in detail.
[0121] The influence of table 1 reaction conditions on intermediate compound B2 yield
[0122]
[0123] From Table 1, the reaction can obtain a relatively ideal reaction yield in a high-boiling solvent. As shown in the above-mentioned examples 1-4, the preferred solvent is toluene, dimethyl sulfoxide, N,N-dimethylformamide, For 1,2-dioxane, when the low boiling point solvent as shown in Comparative Example 4 is used for reaction, the reaction yield decreases significantly. Due to the partial deboronation of 1-alkenylhexylboronic acid in the reaction, when its amount is greater than 1eq of the theoretical value, the reaction yield is ideal, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com