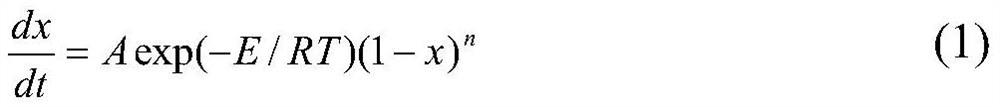Method for rapidly calculating self-accelerated decomposition temperature of substance and application of method
A self-accelerating decomposition and fast calculation technology, which can be used in complex mathematical operations, investigation phase/state changes, material thermal development, etc. To achieve the effect of reducing security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
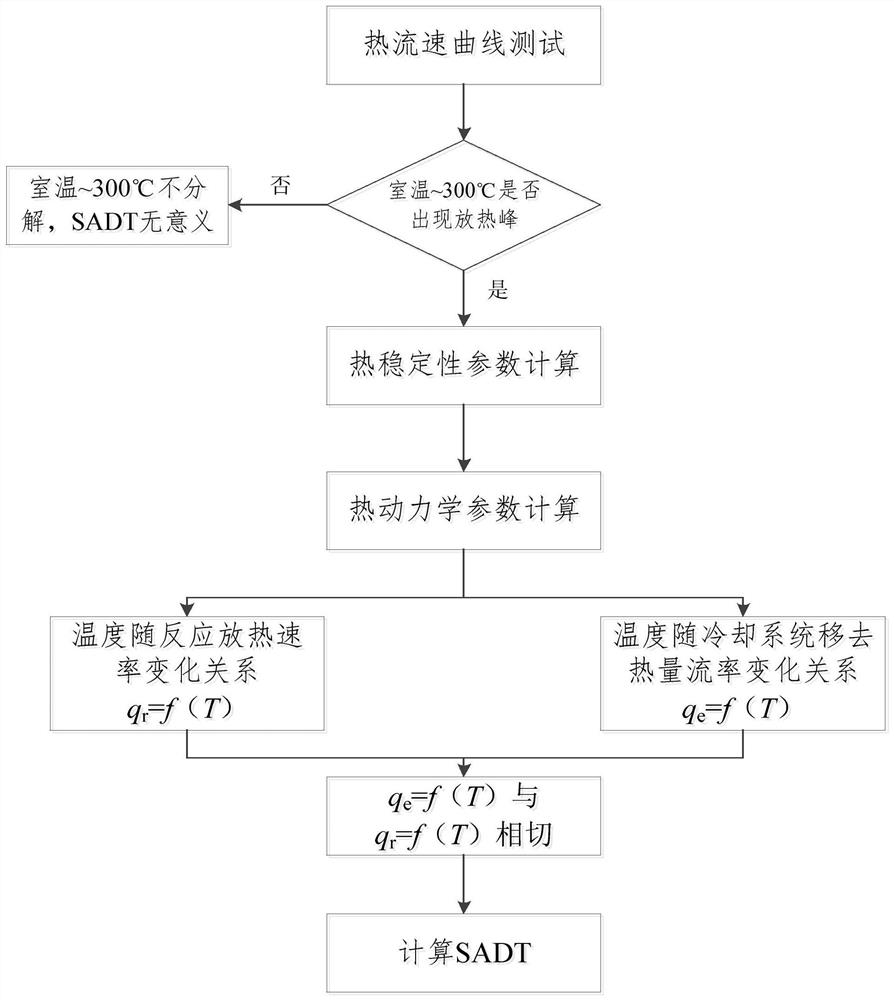
[0075] In this embodiment, differential scanning calorimetry is used to conduct a thermal scanning test on the substance.
[0076] 1.1. Test method:
[0077] The calorimeter is used to conduct a thermal scanning test on the substance in the range of room temperature to 300°C, and the relationship between the heat flow of the substance and the temperature change is calculated by the calorimeter test software.
[0078] Required instruments, equipment and materials:
[0079] Calorimeters (differential scanning calorimeter, microcalorimeter, adiabatic calorimeter, etc.) are commercial instruments and equipment.
[0080] The differential scanning calorimeter refers to ASTM E537-12 "Standard Test Method for Evaluation of Thermal Stability in Chemicals by Differential Scanning Calorimetry".
[0081] The microcalorimeter refers to the test method of the C80 microcalorimeter developed by the French company Setaram (Setaram).
[0082] The adiabatic calorimeter refers to the VSP relie...
Embodiment 2
[0129] In this embodiment, a microcalorimetric method is used to conduct a thermal scanning test on the substance.
[0130] 2.1. Test method:
[0131] The calorimeter is used to conduct a thermal scanning test on the substance in the range of room temperature to 300°C, and the relationship between the heat flow of the substance and the temperature change is calculated by the calorimeter test software.
[0132] Required instruments, equipment and materials:
[0133] Calorimeters (differential scanning calorimeter, microcalorimeter, adiabatic calorimeter, etc.) are commercial instruments and equipment.
[0134] The differential scanning calorimeter refers to ASTM E537-12 "Standard Test Method for Evaluation of Thermal Stability in Chemicals by Differential Scanning Calorimetry".
[0135] The microcalorimeter refers to the test method of the C80 microcalorimeter developed by the French company Setaram (Setaram).
[0136] The adiabatic calorimeter refers to the VSP relief size ...
Embodiment 3
[0183] In this embodiment, the adiabatic calorimetry test is used to perform a thermal scanning test on the substance.
[0184] 3.1. Test method:
[0185] The calorimeter is used to conduct a thermal scanning test on the substance in the range of room temperature to 300°C, and the relationship between the heat flow of the substance and the temperature change is calculated by the calorimeter test software.
[0186] Required instruments, equipment and materials:
[0187] Calorimeters (differential scanning calorimeter, microcalorimeter, adiabatic calorimeter, etc.) are commercial instruments and equipment.
[0188] The differential scanning calorimeter refers to ASTM E537-12 "Standard Test Method for Evaluation of Thermal Stability in Chemicals by Differential Scanning Calorimetry".
[0189] The microcalorimeter refers to the test method of the C80 microcalorimeter developed by the French company Setaram (Setaram).
[0190] The adiabatic calorimeter refers to the VSP relief s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com