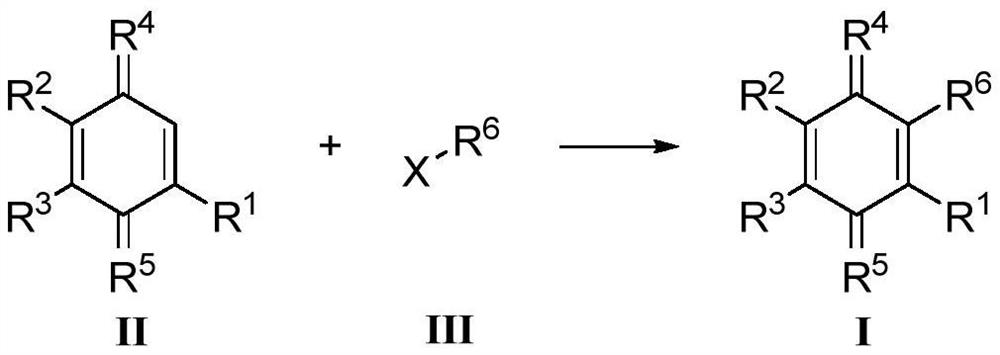
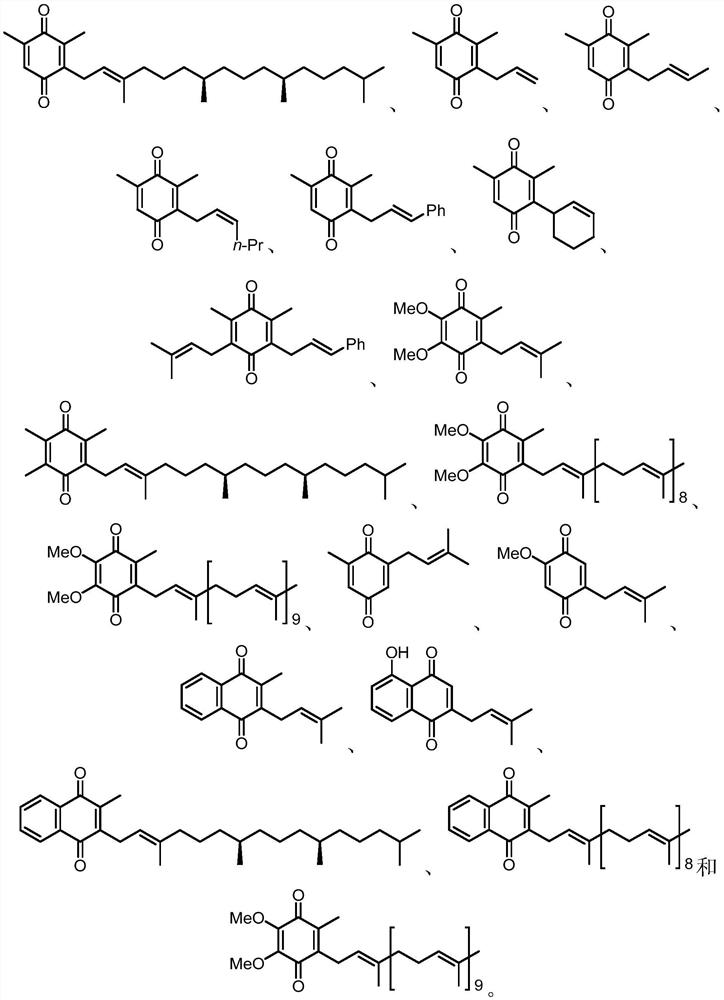
Preparation method of quinone compound
A technology of compounds and quinones, which is applied in the field of preparation of quinones, can solve the problems of single preparation methods of quinones, and achieve the effect of cheap raw materials, easy availability of raw materials, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202]
[0203] In a dry 100mL flask, add a magnetic stirrer, 1.36g (10mmol, 1 equivalent) of 2,3-dimethyl-p-benzoquinone, 2,6-dimethyl-3,5-di(ethoxyacyl )-1,4-dihydropyridine 126mg (0.5mmol, 0.05 equivalent), dry chlorobenzene 50mL, 1-chloro-3-methyl-2-butene (isoamyl chloride) 1.73g (16.5mmol, 1.65 equivalent ), 237 mg (1.5 mmol, 0.15 equivalents) of 2-methyl-2-nonanol. After the solid is dissolved, add 0.8 g of commercially available 60% sodium hydride-mineral oil mixture (about 0.5 g of sodium hydride, about 2 equivalents) to the reaction flask, and then connect an air condenser to the round bottom flask to replace nitrogen And continue to blow nitrogen bubbles in the reaction system. The flask was heated to 80°C and the reaction was stirred for 48 hours. Thereafter, the reaction solution was placed in a centrifuge tube and centrifuged at 10,000 rpm for 2 minutes, and the supernatant was taken and subjected to column chromatography with petroleum ether: ethyl acetate ...
Embodiment 2
[0205]
[0206] In a dry 10mL flask, add a magnetic stirrer, 2,6-dimethyl-p-benzoquinone 68mg (0.5mmol, 1 equivalent), 2,6-dimethyl-3,5-di(ethoxyacyl )-1,4-dihydropyridine 6.3mg (0.025mmol, 0.05 equivalent), dry chlorobenzene 2.5mL, 1-chloro-3-methyl-2-butene (isoamyl chloride) 87mg (0.83mmol, 1.66 equivalent), 2-methyl-2-nonanol 23.7 mg (0.15 mmol, 0.3 equivalent). After the solid dissolves, add 40 mg of commercially available 60% sodium hydride-mineral oil mixture (containing about 24 mg of sodium hydride, 2 equivalents) to the reaction flask, then connect an air condenser to the round bottom flask, replace nitrogen and continue to pass through the flask. Nitrogen bubbles in the reaction system. The flask was heated to 80°C and the reaction was stirred for 48 hours. Thereafter, the reaction solution was placed in a centrifuge tube and centrifuged at 10,000 rpm for 2 minutes, and the supernatant was taken and subjected to column chromatography with petroleum ether: ethyl...
Embodiment 3~31
[0208] After the types and amounts of each raw material in the above-mentioned embodiment 2 were changed, embodiments 3-31 were formed. See the table below for the types and dosage changes of the materials described:
[0209]
[0210]
[0211]
[0212] Note: The equivalents in the above table are based on 2,6-dimethyl-p-benzoquinone as 1 equivalent. HE = 2,6-dimethyl-3,5-di(ethoxyacyl)-1,4-dihydropyridine; HQ = 1,4-hydroquinone.
[0213] K 2 CO 3 The pKa of sodium phenoxide is 10.25, which is slightly higher than that of sodium phenoxide (9.98).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com