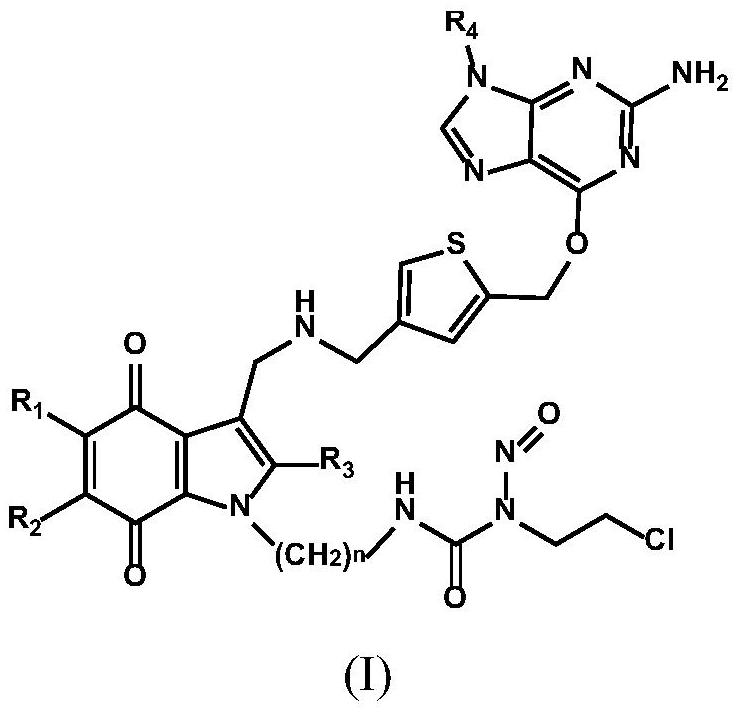
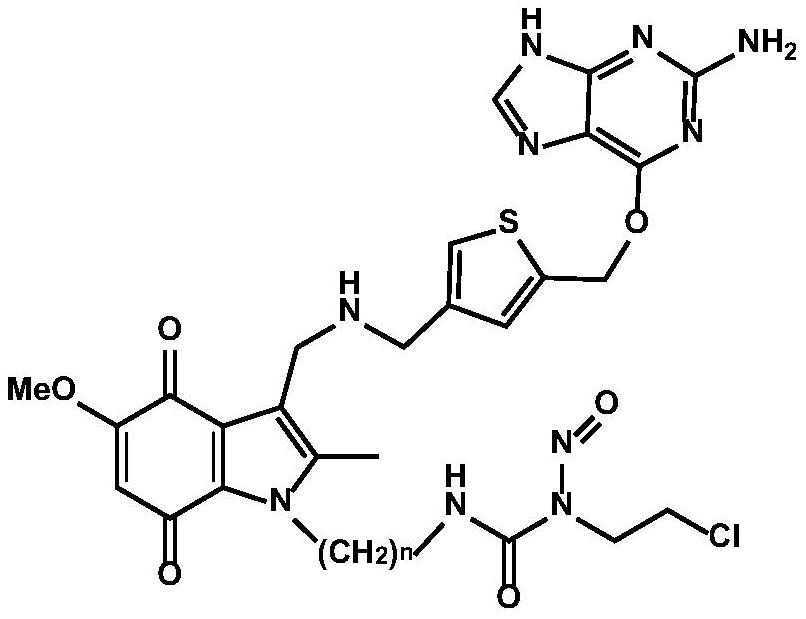
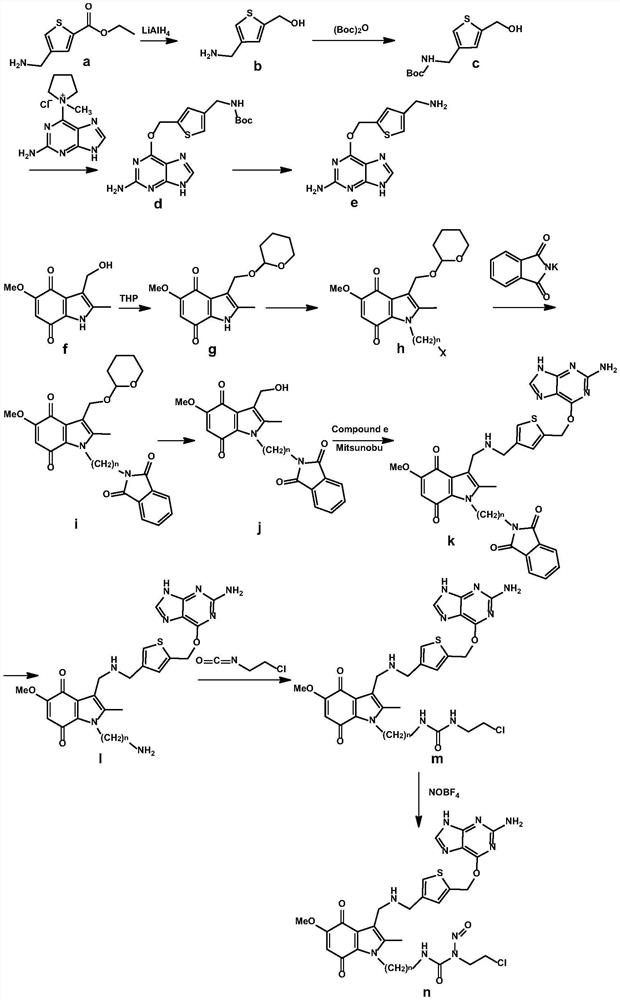
tumor-targeted anti-drug 6 -Thienylguanine-indolequinone-chloroethylnitrosourea combined molecule and preparation method thereof
A chloroethyl nitroso, targeted technology, applied in the direction of antineoplastic drugs, drug combinations, active ingredients of heterocyclic compounds, etc., can solve the problems of lack of tumor targeting and increased toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: 3-(2-(3-((((5-(((2-amino-9H-purin-6-yl)oxy)methyl)thiophen-3-yl)methyl)amino) Methyl)-5-methoxy-2-methyl-4,7-dioxo-2,3,4,7-tetrahydro-1H-indol-1-yl)ethyl)-1-(2 Synthesis of -chloroethyl)-1-nitrosourea (compound 1)
[0069] 1) Synthesis of (4-(aminomethyl)thiophen-2-yl)methanol
[0070] Weigh 4-(aminomethyl)thiophene-2-carboxylate ethyl ester (1.85g, 10mmol) and dissolve it in 10mL of anhydrous THF; weigh out LiAlH 4 (0.2g, 5.2mmol) in a 100mL round bottom flask under N 2 Add 10 mL of anhydrous THF and stir for 10 min under protection and ice bath conditions; add 4-(aminomethyl)thiophene-2-carboxylate ethyl ester solution dropwise, react at 0-10 °C for 2 h; continue to stir in ice bath after the reaction is over , quenched with water, 10% sodium hydroxide solution, water (v / v / v=1:2:3 (water: 0.2 mL)). The reaction solution was concentrated by rotary evaporation under reduced pressure at 50 °C, and then the concentrated solution was separated and purified b...
Embodiment 2
[0129] Example 2: 3-(3-(3-((((5-(((2-amino-9H-purin-6-yl)oxy)methyl)thiophen-3-yl)methyl)amino) Methyl)-5-methoxy-2-methyl-4,7-dioxo-4,7-dihydro-1H-indol-1-yl)propyl)-1-(2-chloroethyl) )-1-Nitrosourea (Compound 2) Synthesis
[0130] 1) Synthesis of (4-(aminomethyl)thiophen-2-yl)methanol
[0131] Weigh 4-(aminomethyl)thiophene-2-carboxylate ethyl ester (2.22 g, 12 mmol) and dissolve it in 10 mL of anhydrous ether; weigh LiAlH 4 (0.25g, 6.5mmol) in a 100mL round bottom flask under N 2 Add 10 mL of anhydrous ether and stir for 10 min under protection and ice bath conditions; add 4-(aminomethyl)thiophene-2-carboxylate ethyl ester solution dropwise, react at 0-10 °C for 2 h; continue to stir in ice bath after the reaction is over was quenched with water, 10% sodium hydroxide solution, water (v / v / v = 1:2:3 (water: 0.25 mL)). The reaction solution was concentrated by rotary evaporation under reduced pressure at 50°C, and then the concentrated solution was separated and purified b...
experiment example 1
[0191] Experimental Example 1: Evaluation of Antitumor Activity
[0192] 1. Experimental materials and instruments
[0193] Test compound: compound 1, compound 2, free carmustine (BCNU) and BCNU+O prepared in the above preparation examples 6 -TMG;
[0194] Cell lines: human glioma cells SF763, SF767, SF126, A549, human breast cancer cells MCF-7, human prostate cancer cells DU145;
[0195] 2. Experimental method
[0196] Six tumor cells were seeded at 1000 / well in 96-well plates at 37°C, 5% CO. 2 After culturing for 24 h, the concentration of BCNU (positive control group 1), BCNU+O 6 -TMG (positive control group 2), compound 1, compound 2, a total of 4 drug treatment groups, each group has 6 duplicate wells, and a control group is set. The CCK-8 solution was used for 4 hours. The above groups were treated under aerobic and hypoxic conditions for 48 h; then, 10 μL of CCK-8 solution was added to each well; finally, the absorbance value at 450 nm was measured. The cell viab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com