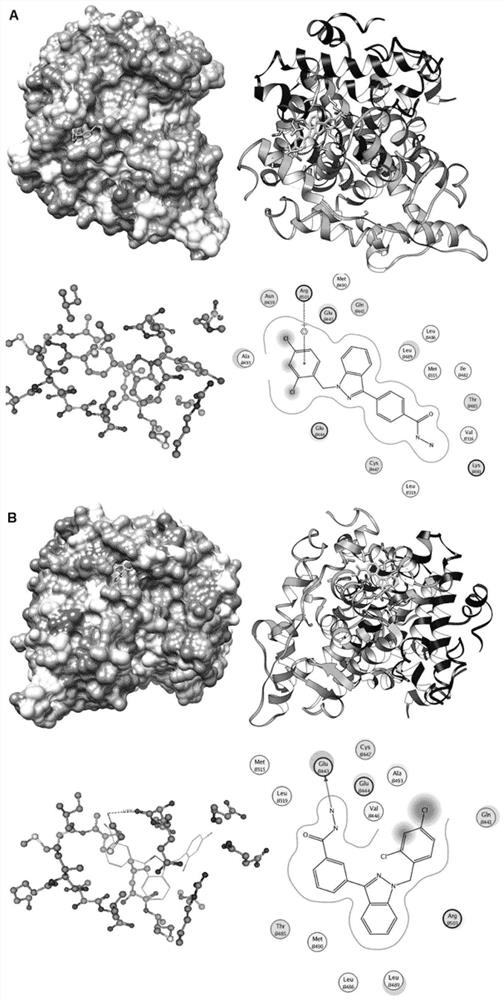
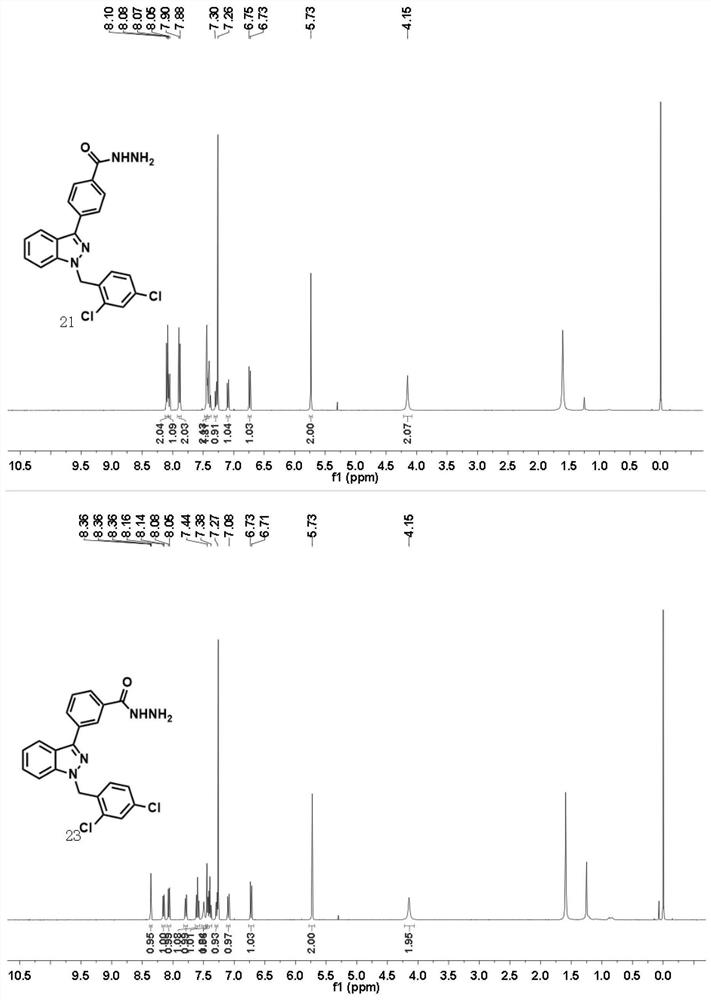
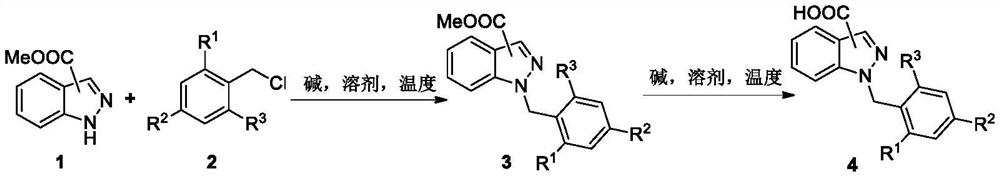
Synthesis method of novel estrogen receptor targeting inhibitor and application of novel estrogen receptor targeting inhibitor in breast cancer treatment
A technology of estrogen receptor and synthesis method, which is applied in the synthesis method of new estrogen receptor targeting inhibitor and its application in the treatment of breast cancer, which can solve the problem of low bioavailability and limited clinical application of lonidamine and other issues to achieve good catalytic effect, cost reduction, and high recycling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Synthesis of 1-(2-chloro-4-fluorobenzyl)-1H-indazole-3-carboxylic acid 4a:
[0264]
[0265] Add 0.20mmol of compound 1a, 0.22mmol of 2a and 0.9mmol of potassium carbonate to the reaction flask in sequence, then add 2mL of acetone, and stir the reaction under reflux at 70°C for 12 hours. After the reaction is completed, add water to quench the reaction, and add 5mL of acetic acid Diluted with ethyl ester, washed with 5 mL of saturated brine, dried the organic phase with anhydrous magnesium sulfate, spin-dried and recrystallized with dichloromethane / n-hexane system to obtain intermediate 3a. Subsequently, the intermediate 3a obtained by recrystallization, 0.40 mmol sodium hydroxide and 2 mL of methanol / water (1:1) mixed solvent were added to the reaction flask, and the reaction was refluxed and stirred at 60 ° C for 24 hours. After the reaction was completed, water was added to quench The reaction was quenched, diluted with 5 mL of ethyl acetate, washed with 5 mL of s...
Embodiment 2
[0268] Synthesis of 1-(2,4-difluorobenzyl)-1H-indazole-3-carboxylic acid 4b:
[0269]
[0270] Add 0.20mmol of compound 1a, 0.22mmol of 2b and 0.9mmol of potassium carbonate into the reaction flask in sequence, then add 2mL of acetone, and stir the reaction under reflux at 70°C for 12 hours. After the reaction is completed, add water to quench the reaction, add 5mL of Diluted with ethyl acetate, washed with 5 mL of saturated brine, dried the organic phase with anhydrous magnesium sulfate, spin-dried and recrystallized with dichloromethane / n-hexane system to obtain intermediate 3b. Subsequently, the intermediate 3b obtained by recrystallization, 0.40 mmol potassium hydroxide and 2 mL methanol / water (1:1) mixed solvent were added to the reaction flask, and the reaction was refluxed and stirred at 60 ° C for 24 hours. After the reaction was completed, water was added to quench The reaction was quenched, diluted with 5 mL of ethyl acetate, washed with 5 mL of saturated brine, d...
Embodiment 3
[0273] Synthesis of 1-(2,6-dichlorobenzyl)-1H-indazole-3-carboxylic acid 4c:
[0274]
[0275] Add 0.20mmol of compound 1a, 0.22mmol of 2c and 0.9mmol of sodium carbonate to the reaction flask in sequence, then add 2mL of acetone, and stir the reaction under reflux at 70°C for 12 hours. After the reaction is over, add water to quench the reaction, add 5mL of Diluted with ethyl acetate, washed with 5 mL of saturated brine, dried the organic phase with anhydrous magnesium sulfate, spin-dried and recrystallized with dichloromethane / n-hexane system to obtain intermediate 3c. Subsequently, the intermediate 3c obtained by recrystallization, 0.40 mmol sodium hydroxide and 2 mL of ethanol / water (1:1) mixed solvent were added to the reaction flask, and the reaction was refluxed and stirred at 60 ° C for 24 hours. After the reaction was completed, water was added to quench The reaction was quenched, diluted with 5 mL of ethyl acetate, washed with 5 mL of saturated brine, dried with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com