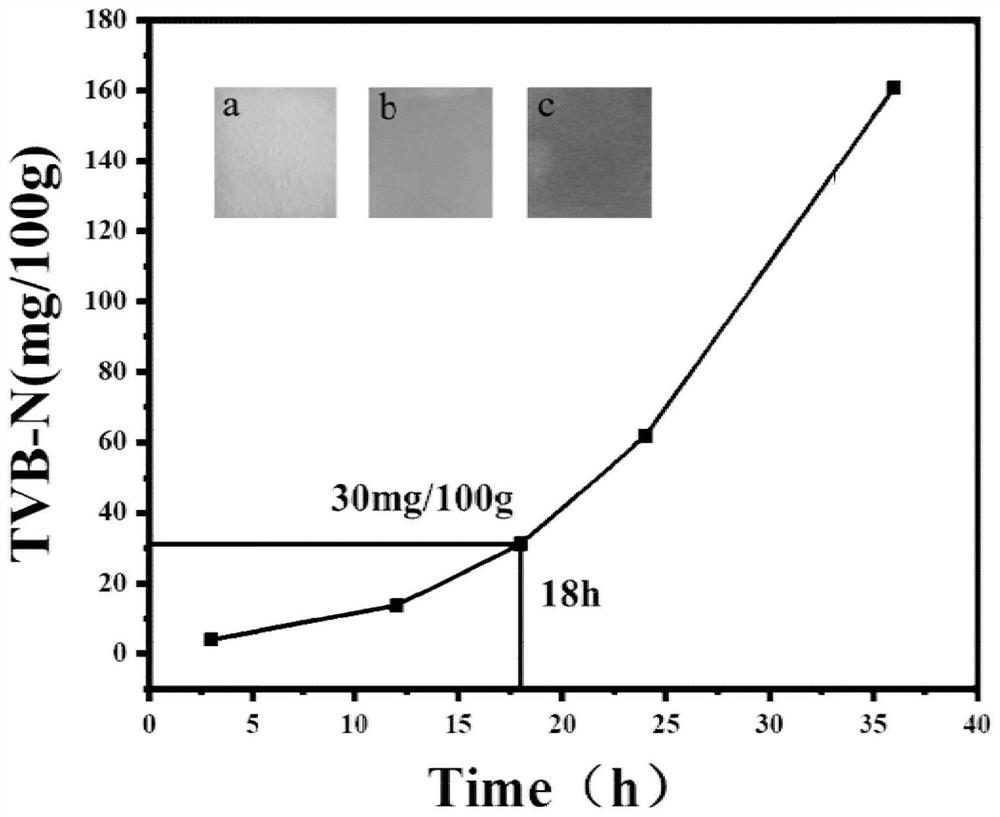
Volatile basic nitrogen response type compound, portable test paper, preparation method and application
A volatile base nitrogen, portable detection technology, applied in the field of analysis and detection, can solve the problems such as not intuitive response in the spoilage process of aquatic products, cumbersome indicator test paper making process, poor chemical stability of the indicator, etc. The effect of good application potential and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
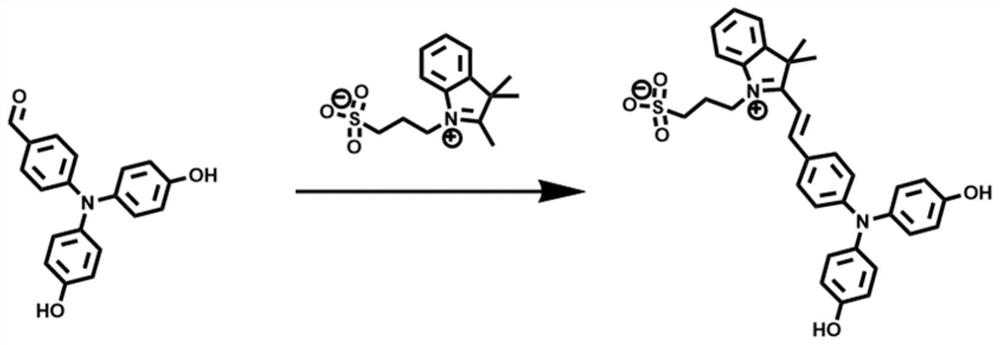
[0039] Take 4-(bis(4-hydroxyphenyl)amino)benzaldehyde 305.11mg (1mmol) and 3-(2,3,3-trimethyl-3H-indole-1-salt-yl)propane-1- Add 562.22 mg (2 mmol) of sulfonate into 15 mL of absolute ethanol, then add 100 μL of piperidine dropwise to the solution, vacuumize the system and fill it with nitrogen gas and repeat 3 times, heat up to 70 ° C and stir for 4 hours, and the solution after reaction Cool to room temperature, remove the solvent by distillation under reduced pressure, dissolve in methanol and add dropwise into anhydrous diethyl ether at 8°C to precipitate a solid, wash with anhydrous diethyl ether three times, the amount of anhydrous diethyl ether is 40mL each time, to obtain 3-(2 -(4-(bis(4-hydroxyphenyl)amino)styryl)-3,3-dimethyl-3H-indol-1-salt-1-yl)propane-1-sulfonate 300mg( Yield: 52.80%).
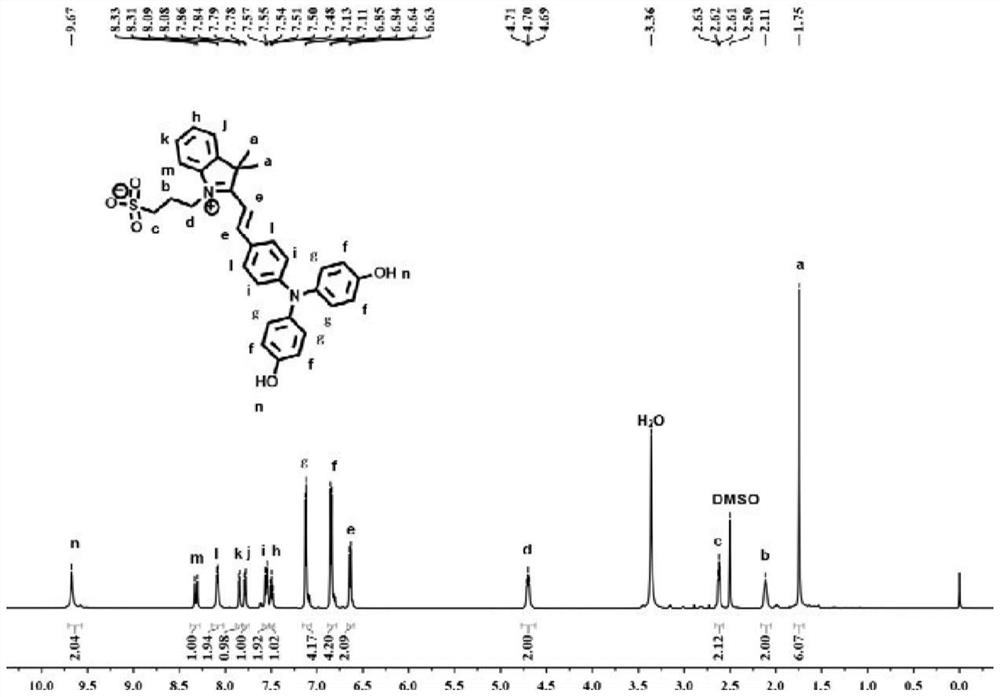
[0040] The resulting product was characterized by proton nuclear magnetic resonance spectroscopy: 1 H NMR (600MHz, DMSO-d 6 )δ9.67(s,2H),8.32(d,J=15.7Hz,1H),8.09(d,J=8.2Hz,2H),...
Embodiment 2
[0043] Take 4-(bis(4-hydroxyphenyl)amino)benzaldehyde 305.11mg (1mmol) and 3-(2,3,3-trimethyl-3H-indole-1-salt-yl)propane-1- Add 702.78mg (2.5mmol) of sulfonate into 20mL of absolute ethanol, then dropwise add 125μL of piperidine to the solution, vacuumize the system and fill it with nitrogen and repeat 3 times, heat up to 75°C and stir for 5 hours, and the reacted The solution was cooled to room temperature, and after the solvent was distilled off under reduced pressure, it was dissolved in methanol and added dropwise into anhydrous diethyl ether at 6°C to precipitate a solid, which was washed three times with anhydrous diethyl ether, and the amount of anhydrous diethyl ether was 45 mL each time to obtain 3-( 2-(4-(bis(4-hydroxyphenyl)amino)styryl)-3,3-dimethyl-3H-indol-1-salt-1-yl)propane-1-sulfonate 315mg (Yield: 55.44%).
[0044] The characterization results of the compounds obtained in this example are the same as those in Example 1.
Embodiment 3
[0046] Take 4-(bis(4-hydroxyphenyl)amino)benzaldehyde 305.11mg (1mmol) and 3-(2,3,3-trimethyl-3H-indole-1-salt-yl)propane-1- Add 844.33 mg (3 mmol) of sulfonate into 25 mL of absolute ethanol, then add 150 μL of piperidine dropwise into the solution, vacuumize the system and fill it with nitrogen gas and repeat 3 times, heat up to 80°C and stir for 6 hours, and the solution after reaction Cool to room temperature, remove the solvent by distillation under reduced pressure, dissolve in methanol and add dropwise into anhydrous diethyl ether at 4°C to precipitate a solid, wash with anhydrous diethyl ether three times, the amount of anhydrous diethyl ether is 50mL each time, to obtain 3-(2 -(4-(bis(4-hydroxyphenyl)amino)styryl)-3,3-dimethyl-3H-indol-1-salt-1-yl)propane-1-sulfonate 340mg( Yield: 59.84%).
[0047] The characterization results of the compounds obtained in this example are the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com