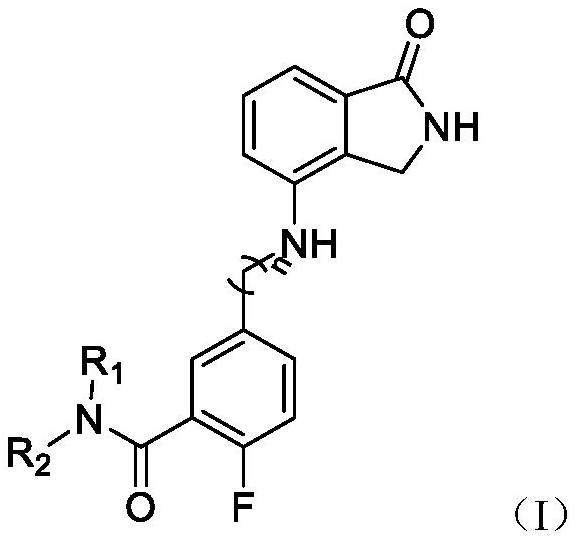
Isoindolone compound as well as preparation method and application thereof
A compound and composition technology, applied in the field of medicine, can solve problems such as hindering development, achieve the effects of alleviating toxic and side effects, effectively inhibiting the activity of PARP1 protease, and solving the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
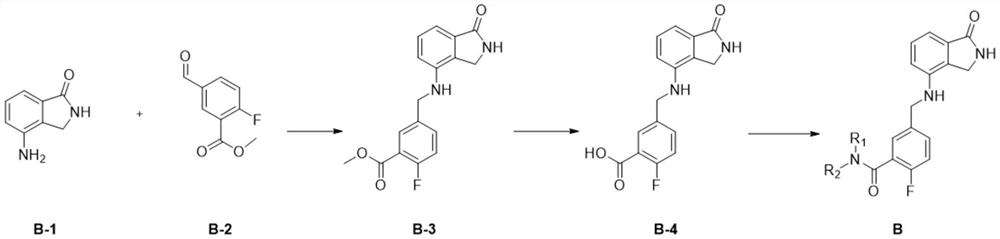
Image
Examples
Embodiment 1
[0049] Preparation of target product compound 1
[0050]
[0051] The preparation steps are as follows:
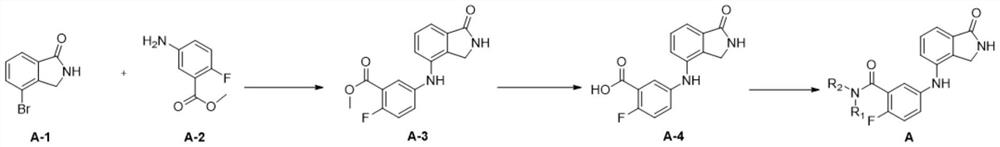
[0052] 1) Preparation of compound A-3
[0053]
[0054] 4-Bromoisoindolin-1-one (743 mg, 3.50 mmol) was dissolved in dioxane (35.0 mL) and H 2 O (3.50mL), were added 5-amino-2-fluorobenzoic acid methyl ester (800mg, 4.73mmol), Pd 2 (dba) 3 (160mg, 0.180mmol), K 3 PO 4 (2.60g, 12.3mmol) and brettphos (188mg, 0.350mmol) were reacted at 100°C for 15h. After the reaction solution was cooled to room temperature, it was filtered with diatomaceous earth, washed with ethyl acetate, dried and concentrated, and purified by column chromatography (dichloromethane:methanol=20:1) to obtain compound A-3 (white solid, 840mg, 80%) .
[0055] Compound A-3 was detected, and the detection results were as follows: 1 H NMR (400MHz, DMSO-d 6 )δ8.52(s,1H),8.13(s,1H),7.56(dd,J=6.1,3.0Hz,1H),7.41–6.83(m,5H),4.27(s,2H),3.85(s ,3H). 13 CNMR (100MHz, DMSO) δ170.6, 164.6, 164.5, 155.8 ...
Embodiment 2
[0063] Preparation of target product compound 2
[0064]
[0065] The preparation steps are as follows:
[0066] 1) Preparation of compound A-3: The preparation steps of A-3 in Example 1 were the same to obtain compound A-3.
[0067] 2) Preparation of compound A-4: The preparation steps of A-4 in Example 1 were the same to obtain compound A-4.
[0068] 3) Preparation of Compound 2
[0069] On the basis of Example 1, the difference from Example 1 is that the amine used is different, and what is used in this example is cyclobutyl (piperazin-1-yl) ketone (27.4mg, 0.170mmol), according to The synthesis steps described in Example 1 obtained the target compound 2. Flash column chromatography (dichloromethane:methanol=20:1) gave compound 2 (53.4 mg, 72%, white solid).
[0070] Compound 2 was detected, and the detection results were as follows: 1 H NMR (400MHz, Chloroform-d) δ8.17(s,1H),7.89(d,J=8.5Hz,1H),7.83(q,J=8.9Hz,1H),7.67–7.56(m,2H) ,7.49–7.43(m,2H),7.15–6.92(m,1H),4.6...
Embodiment 3
[0072] Preparation of target product compound 3
[0073]
[0074] The preparation steps are as follows:
[0075] 1) Preparation of compound A-3
[0076] The preparation steps of A-3 in Example 1 were the same to obtain compound A-3.
[0077] 2) Preparation of Compound A-4
[0078] The preparation steps of A-4 in Example 1 were the same to obtain compound A-4.
[0079] 3) Preparation of compound 3
[0080] On the basis of Example 1, the difference from Example 1 is that the amines used are different, and what is used in this example is cyclopentyl (piperazin-1-yl) ketone (28.8 mg, 0.170 mmol), according to The synthesis steps described in Example 1 obtained the target compound 3. Flash column chromatography (dichloromethane:methanol=20:1) gave compound 3 (53.6 mg, 70%, white solid).
[0081] Compound 3 was detected, and the detection results were as follows: 1 H NMR (400MHz, Chloroform-d) δ8.11(s,1H),7.99(d,J=7.5Hz,1H),7.81(q,J=8.9Hz,1H),7.67–7.56(m,2H) ,7.48–7.43(m,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com