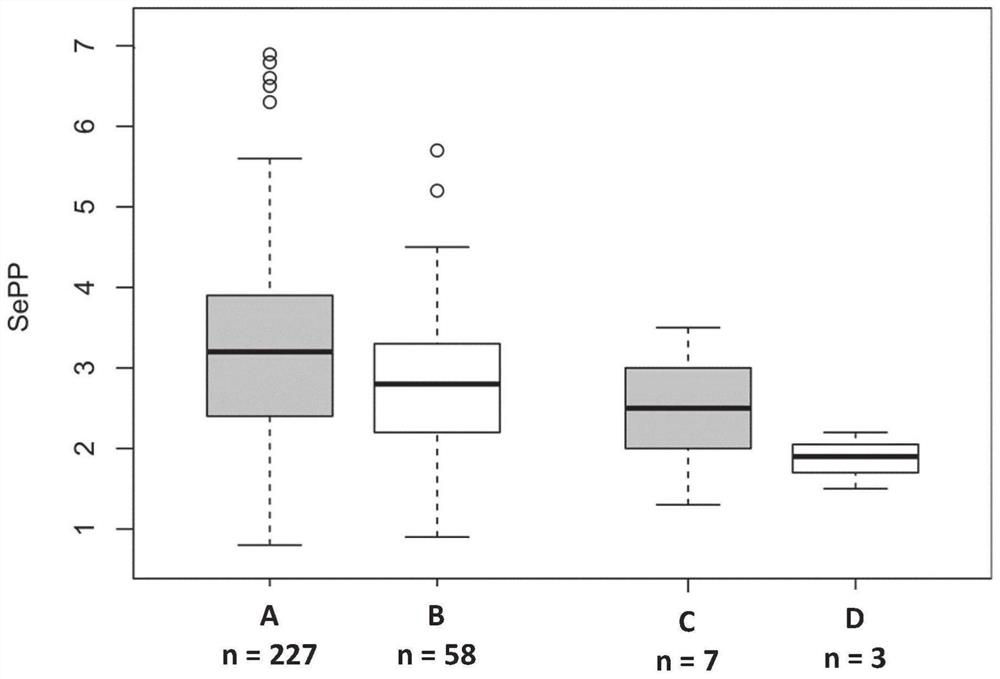
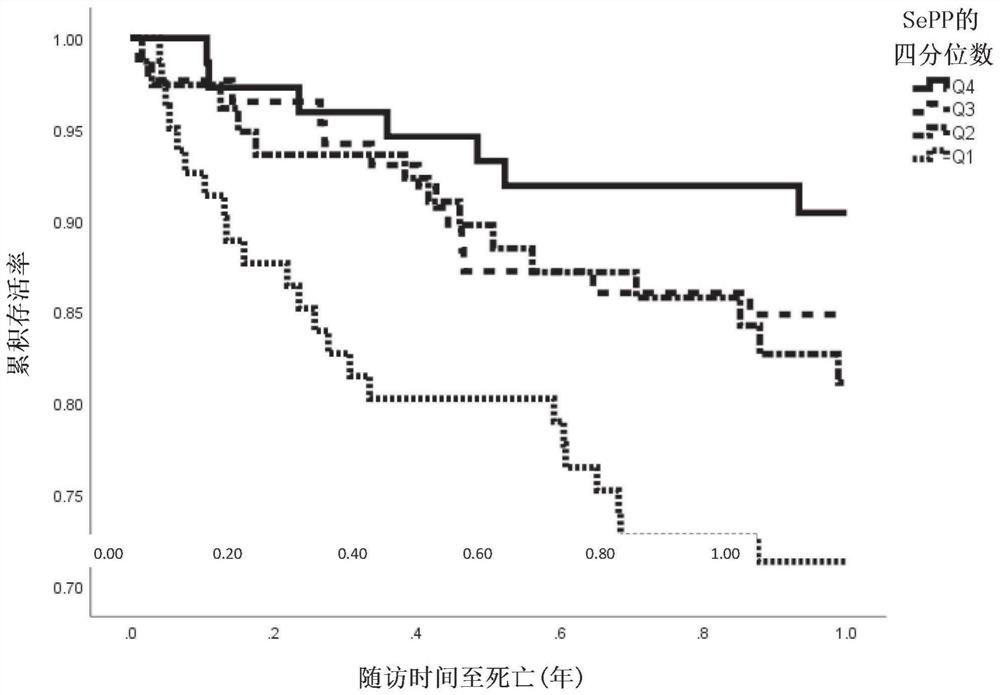
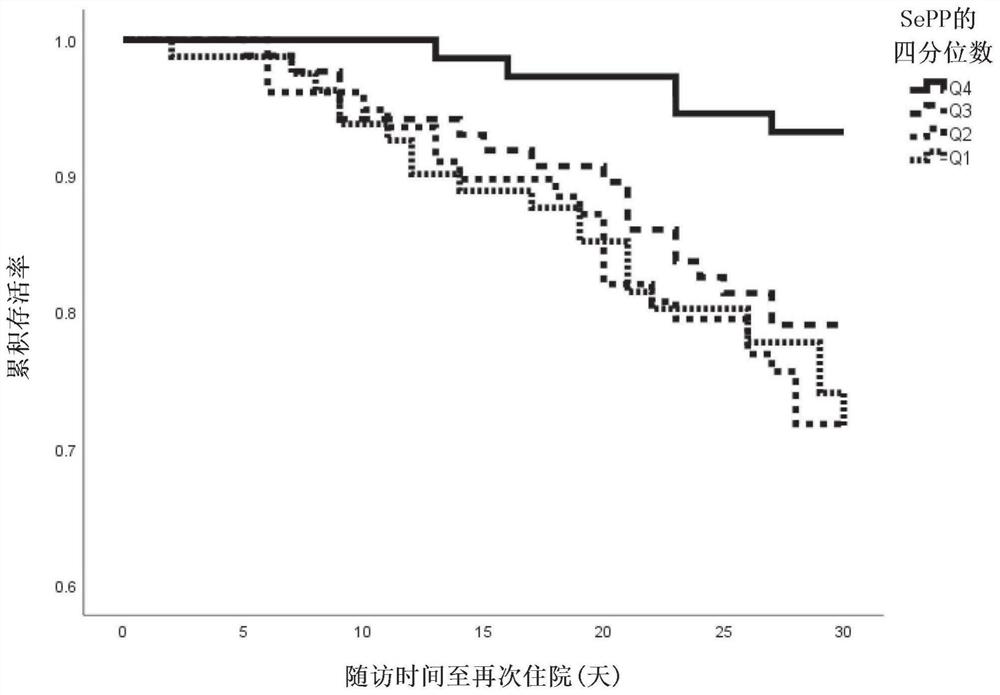
Selenoprotein p in heart failure
A heart failure and selenoprotein technology, which is applied in cardiovascular system diseases, sulfur/selenium/tellurium active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as research on selenoprotein associations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Example 1: Assay Description
[0162] Selenotest ELISA ( Hybsier et al., 2017. Redox Biology 11:403-414; Hybsier et al., 2015. Perspectives in Science 3:23-24 ), a chromogenic enzyme-linked immunosorbent assay for the quantitative determination of the human selenoprotein selenoprotein P in serum samples. The Selenotest ELISA is a sandwich enzyme immunoassay performed in a 96-well plate format and uses two different selenoproteins, selenoprotein P-specific monoclonal antibodies, for the antigen capture and detection steps. Selenoprotein P levels were determined for calibrators and controls by measuring against serial dilutions of the NIST SRM 1950 standard reference material. Monoclonal antibodies (Ab) were produced by immunizing mice with an emulsion of purified recombinant selenoprotein P. The specific monoclonal antibody Ab5 was immobilized as the capture antibody, and the specific mAb2 was used as the detection antibody. The lower limit of quantitation was dete...
Embodiment 2
[0163] Example 2: HARVEST- Research
[0164] The Swedish Heart Failure Survey Study (HARVEST- ) is a Swedish Prospective, ongoing study of consecutive patients hospitalized for acute heart failure (newly diagnosed or worsening chronic heart failure). The only exclusion criterion was inability to express consent. Baseline data, including blood donation and clinical examination, were collected for 324 subjects between March 2014 and September 2018. Complete data were available for 295 patients. Data on one-year mortality (54 events), one-year cardiovascular-related mortality (44 events), and 30-day readmission (61 events) were retrieved from national and regional registries. Selenoprotein P was measured on admission, as well as a clinical examination.
[0165] clinical examination
[0166] After hospitalization, fasting blood samples were drawn, blood pressure was measured, and body mass index (BMI) was calculated in kilograms per square meter. Subjects' health status ...
Embodiment 3
[0225] Example 3: MPP Study
[0226] research description
[0227] group based The Prevention Project (MPP) is based on a Swedish single-center prospective cohort study. Between 1974 and 1992, a total of A total of 33,346 men and women of the same ethnic background in an urban area were screened for traditional risk factors for all-cause mortality and cardiovascular disease (CVD). A detailed description of the baseline procedure can be found elsewhere ( Fedorowski et al., 2010. Eur Heart J 31:85-91; Berglund et al., 1996 . J Intern Med 239:489-97 ). Between 2002 and 2006, all survivors of the original MPP cohort were invited to undergo re-examination. Of these, 18,240 participants (n = 6,682 women) responded to the invitation and underwent re-examination, including blood sampling and immediate -80°C storage of EDTA plasma aliquots. The 2002-2006 re-examination represents the baseline time point for the current study.
[0228] A random sample of 5,060 of 18,240 sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com