Stem cell medicine for treating diabetes
A technology of stem cells and diabetes, applied in the field of biomedicine, can solve the problem of decreased sensitivity of insulin signaling pathway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
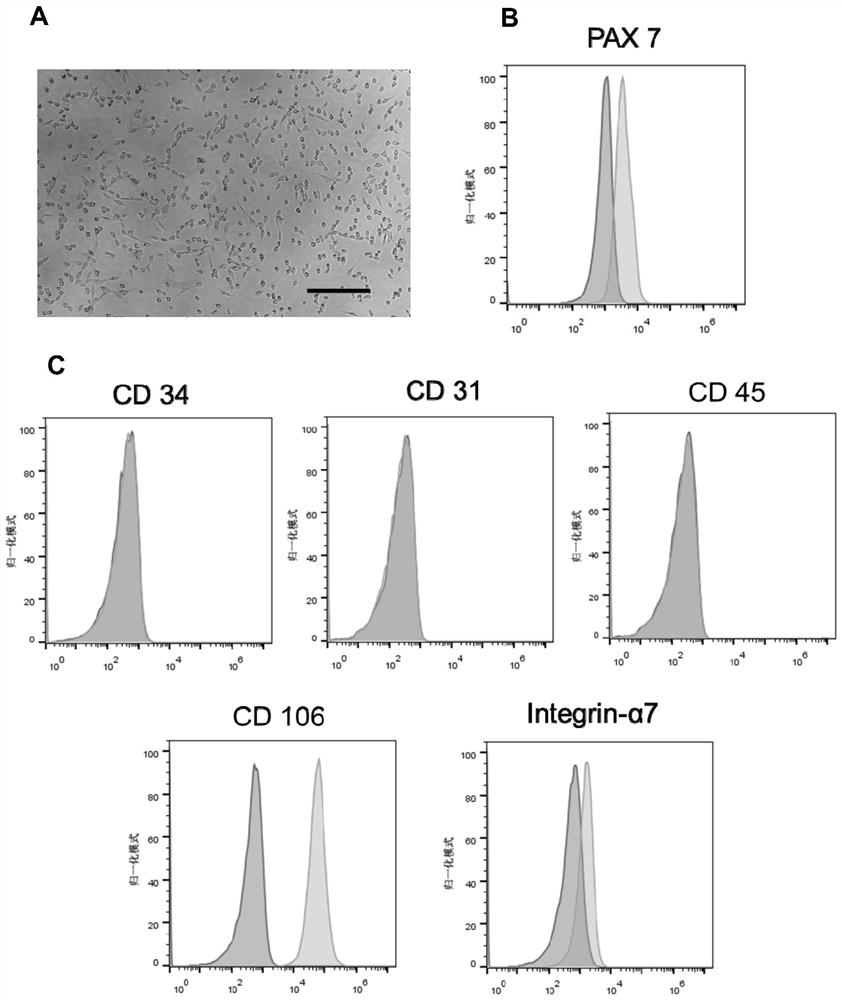
[0208] Example 1 Extraction, Expansion, Identification and Differentiation of Mouse Muscle Stem Cells
[0209] (1) Extraction of mouse-derived muscle stem cells
[0210] 1. Take the mouse hind leg muscle tissue, cut it into pieces to form a tissue slurry, add 10mL type II collagenase (750U / mL), put it in a constant temperature shaker, incubate at 37°C for 60min, and the shaker speed is 70rpm.
[0211] 2. After incubation and digestion, use complete medium (containing 10% FBS) to neutralize, and centrifuge to remove the supernatant.
[0212]3. Add 10 mL of mixed digestion solution of type II collagenase (100 U / mL) and dispase (1.1 U / mL) to the pelleted cell mass again, and resuspend the cell pellet.
[0213] 4. Put the cell suspension into the constant temperature shaker again to incubate and digest. The incubation condition is 37°C for 60min, and the shaker speed is 70rpm.
[0214] 5. After the incubation, add neutralizing solution to stop the digestion, centrifuge the resul...
Embodiment 2
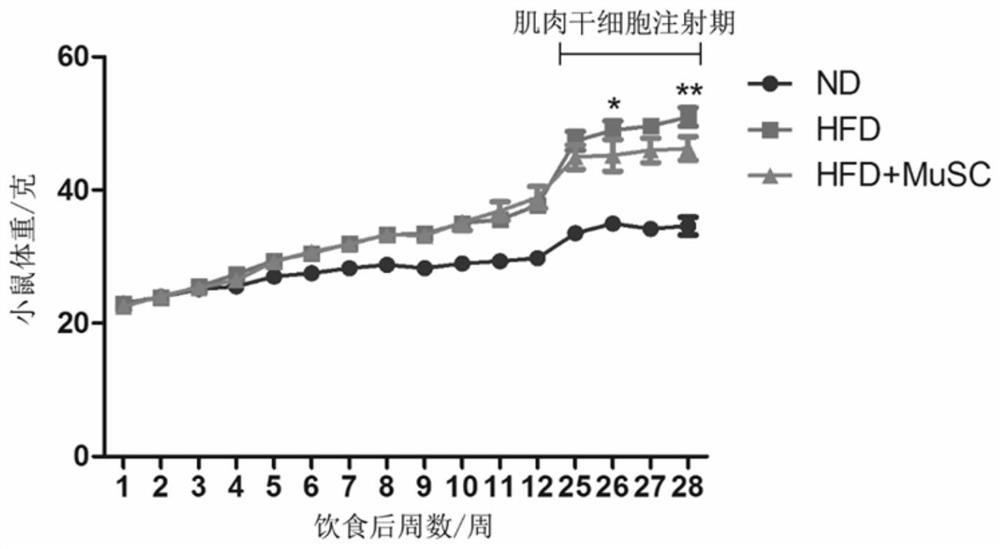
[0225] Example 2 Mouse high-fat diet-induced insulin resistance model and transplantation of mouse muscle stem cells
[0226] (1) Establishment of mouse model
[0227] 4-week-old male C57 mice were randomly selected and divided into groups, and fed with a normal diet containing 10% Kal fat and a high-fat diet containing 60% Kal fat, respectively. The body weight of the mice was recorded every day to form a body weight change curve. After 2-3 months of modeling, mice with a body weight of about 40 grams were selected for the experiment. High-fat diet-fed mice develop insulin resistance due to obesity and can be used as a model of type 2 diabetes.
[0228] (2) In vivo transplantation of mouse-derived muscle stem cells
[0229] 1. After the model is established, the mice are divided into experimental groups, which are divided into control group (normal diet group), model group (high-fat diet group) and treatment group (high-fat diet mouse group injected with mouse muscle stem ...
Embodiment 3
[0233] Example 3 Regulatory Effect of Mouse-derived Muscle Stem Cells on Glucose Metabolism
[0234] (1) Detection of Glucose Tolerance (GTT)
[0235] 1. Fifteen hours before the test, the mice were given fasting and water treatment.
[0236] 2. Detect the fasting blood glucose of the mice with a Bino blood glucose meter and blood glucose test strips, and record it as G0.
[0237] 3. Administer glucose to the mouse intraperitoneally, and the injection amount is 1g / g mouse. Immediately after the injection, the time at this moment was recorded as T0.
[0238] The mouse blood glucose was measured again after 15min (T15), 30min (T30), 45min (T45), 60min (T60), and 120min (T120), and recorded as G15, G30, G45, G60, G120 respectively.
[0239] (2) Detection of insulin resistance (ITT)
[0240] 1. Use the Bino blood glucose meter and blood glucose test strips to detect the fasting blood glucose of the mice, and record it as G0.
[0241] 2. Give the mice intraperitoneal injection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com