Biomarker detection kit for cholestasis indication prognosis
A technology for prognostic markers, cholestasis, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Research Object Determination, Followup and Packet
[0033] 1.1 research object
[0034] Fudan University of Fudan University and its medical affiliated members of Fudan University Affiliated, Jinshan Hospital, clinical and clinical, and gene diagnosis, and gene diagnosis of JAG1-mutation caused by blood specimens. During the 2015 / 01 / 01-2017 / 12 / 30 period, the specimen is discovered, and the specimen is retained as the verification set during 2018 / 01 / 01-2020 / 10 / 31. The Algs diagnostic criteria are as follows: no family history, via pathologically confirmed the lack of bile ducts in liver, in line with cardiac, eye abnormal eye (corneal embryo), butterfly vertebrae, nephrotomy and special faces in 5 items in 5 Diagnosis; no family history, no liver pathological examination, in line 4 or more people can be diagnosed; there is 1 JAG1 gene mutator in compliance with 1 of the above 5 items.
[0035] 1.2 follow-up and group
[0036] The clinical data of children co...
Embodiment 2
[0041] Example 2 Study object specimen processing and analysis
[0042] 2.1 Specimen Processing (1) Discovering Excavation / Plasma lyophilized specimens:
[0043] Serum / plasma 100 μl, dried in vacuo, and stored in a -80 ° C refrigerator, and the UPLC / MRM-MS technology was quantified after treatment.
[0044] S1 extraction bile acid: take out 100 μL of serum / plasma lyophilized specimens, add LC-MS grade water (H 2 O) 100 μL dissolved, and then put it in the ultrasonic oscillator for 15 minutes, apply a vortex to 15 minutes, so that serum / plasma solute is fully dissolved; 30 μL of the new EP tube is taken, add pre-formulated acetonitrile: methanol volume ratio 1 : 1 mixed solution 90 μL (water: organic solvent to maintain 1: 3 ratio), vortex for 10 seconds, ice water in ice water 120 seconds, repeating the above vortex and ultrasonic oscillating step three times to make the solute fully dissolved, then high-speed low temperature, centrifugation conditions It was 15000 rpm, ...
Embodiment 3
[0087] Example 3 results analysis discussion
[0088] 3.1 Population Characteristics
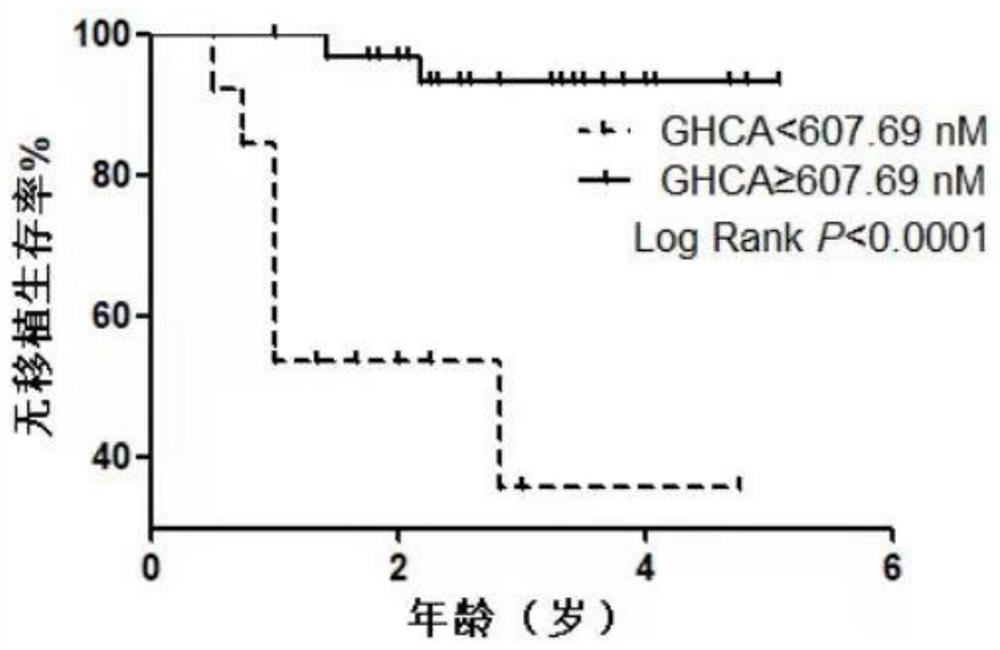
[0089] A total of 21 JAG1 gene mutations and clinical diagnosis of ALGS were incorporated into the blood specimen discovery (Table 2), including 10 cases of Poor Prognos, male 5 cases; good prognosis 11 cases, male 6 For example, there were no statistical differences in gender (P = 0.771). In the 10 cases of the child, 2 patients died during the age of 10, 4 cases were left and right, and 4 patients were followed up to 2 years old and 1st to 3 years old, still continued to severe jaundice. 11 patients with preliminary children, 5 cases of light jaundice, 6 patients with jaundice fell back. The pre-prognosis is 2.83 (1.83, 4.00) elder than the prognosis of children 1.80 (1.00, 2.44) year (P = 0.003), may be terminated by liver transplant surgery or death due to 6 patients in the prognosis group. Follow-up. The selection specimens of the two groups were 1 year before the jaundice did not completel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com