Synthesis method of key intermediate of immunomodulator
An immunomodulator and synthesis method technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylates, etc., can solve the problems of application impact, residual metal substances, insufficient purity of the final product, etc., and reduce metal residues. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of compound 4, its reaction formula is as follows:
[0030]
[0031] 1.2eq of compound 2 and 340mg of compound 4 were dissolved in 100mL of ethanol, 5 equivalents of potassium carbonate was added, nitrogen was replaced 3 times, 2mol% S-Phos was added under the protection of nitrogen, and the temperature was raised to 40°C for 12 hours. After the reaction, cool down to room temperature, filter, concentrate, add water and dichloromethane, wash the organic phase with dilute hydrochloric acid, and dry over anhydrous sodium sulfate. The crude product was purified by column chromatography to obtain 355 mg with a yield of 84%.
Embodiment 2
[0033] The preparation of compound 5, its reaction formula is as follows,
[0034]
[0035] 400mg of compound 4 was dissolved in tetrahydrofuran, added 1.5eq N, O-dimethylhydroxylamine hydrochloride and 2eq triethylamine, 1.8eq EDCI. After stirring at room temperature for 12 hours. Water was added to quench the reaction, then dichloromethane was added and washed with aqueous ammonium chloride. The organic phase was dried over anhydrous sodium sulfate and concentrated three times with dry tetrahydrofuran. To the resulting tetrahydrofuran solution was added DIBAL (diisobutylaluminum hydride) at -78°C. After the reaction was completed, an aqueous solution of potassium bisulfate was added below 0°C. After the resulting mixture was filtered, dichloromethane and 1N hydrochloric acid were added to wash. The obtained organic phase was concentrated to dryness and directly sent to the next step without purification.
Embodiment 3
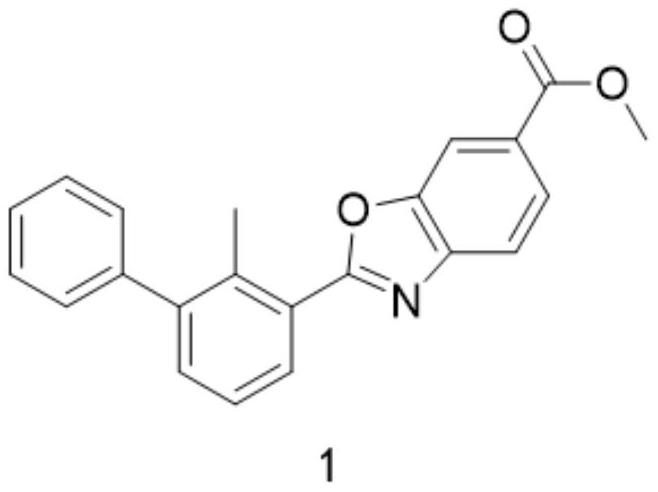
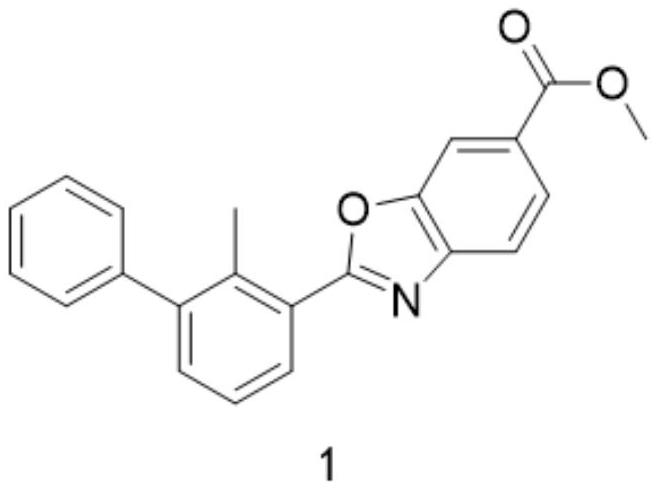
[0037] The preparation of compound 1, its reaction formula is as follows:
[0038]
[0039] 1.5eq of compound 5 and 167mg of compound 6 were mixed in dry dichloromethane, and after 30 minutes of reaction, 2eq of Dess-Martin oxidation reagent (1,1,1-Triacetoxy-1,1-dihydro-1,2-benzodoxol-3 (1H)-one). After the detection reaction was completed, compound 1 was obtained only through silica gel column purification with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com