Method for determining three active components in medicinal preparation
A technology of pharmaceutical preparations and active ingredients, applied in the field of quality control and component testing of traditional Chinese medicine, can solve the problems of lack of effective quality control of pharmaceutical preparations, achieve the effects of improving safety and reliability, broad application prospects, and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
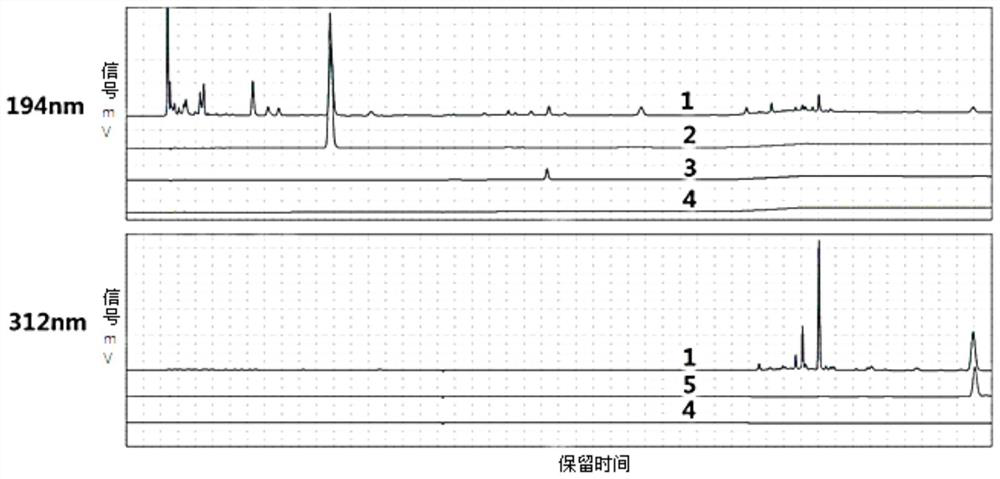
Image
Examples
Embodiment 1
[0060] 1. Preparation of the test solution
[0061] The pharmaceutical preparation samples were prepared according to the ancient method recorded in the classic famous prescription "Synopsis of the Golden Chamber", that is, the fresh lilies were washed with clean water to remove the floating water on the surface, and 7 lilies were weighed. Wash it with clean water and set it aside; take fresh rehmannia glutinosa and squeeze 200mL of fresh rehmannia glutinosa juice into a juicer and boil it for later use. Take the above-mentioned lilies, add 400mL of water to a beaker, place them in a medicine pot, heat and decoct to 200mL, filter, add the above-mentioned fresh rehmannia juice to the filtrate, stir evenly, heat and decoct to 300mL, and the product is ready.
[0062] Take 3g of the drug preparation sample, accurately weigh it, put it in a 50ml measuring bottle, add water to dissolve, and dilute to the scale constant volume, shake well, and filter through a 0.45μm microporous mem...
Embodiment 2
[0079] Methodological verification was carried out on the detection methods of catalpol, diglucoside D, and lilyoside B in the pharmaceutical preparation of the present invention, and the performance index results are as follows.
[0080] 1. Precision
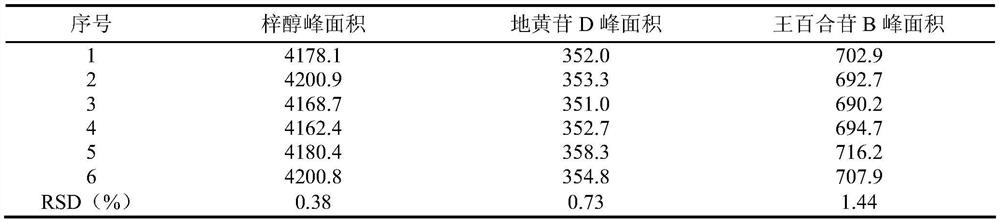
[0081] Get pharmaceutical preparation sample, prepare and obtain need testing solution by step 1 in embodiment 1, press the chromatographic condition of step 3 in embodiment 1, continuous sampling analysis 6 times, measure peak area, precision specific result is shown in Table 2. The results showed that the chromatographic peak area RSDs of catalpol, diglucoside D, and lilyoside B were all less than 2.0%, which indicated that the precision of the method was good.
[0082] Table 2
[0083]
[0084] 2. Repeatability
[0085] Get the same batch of pharmaceutical preparation samples, prepare 6 parts of need testing solution in parallel according to step 1 in embodiment 1, press the chromatographic condition in step 3 in embodi...
Embodiment 3
[0109] According to 32 parts of pharmaceutical preparation samples prepared by the classic famous prescription "Synopsis of the Golden Chamber" ancient recipe, step 1 in Example 1 is used to prepare the solution for the test product, and by the chromatographic conditions of step 3 in Example 1, detect, the result As shown in Table 9. The results show that the method of the invention can measure the content of ingredients in different pharmaceutical preparations.
[0110] Table 9
[0111]
[0112] According to the requirements of the quality standards for medicinal materials established by research: fresh rehmannia glutinosa contains not less than 1.0% and 0.10% rehmanniaside D, respectively, and the average water content is 76%; The amount of water is 67%. The amount of feed and the transfer rate of each step of the preparation process are shown in Table 10 below.
[0113] Table 10
[0114]
[0115] According to the calculation: based on the average dosage, the amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com