Prostate specific membrane antigen binding ligand conjugate and application thereof
A technology of conjugates and ligands, applied in the medical field, can solve problems such as high renal toxicity, short biological half-life, and low complete remission rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
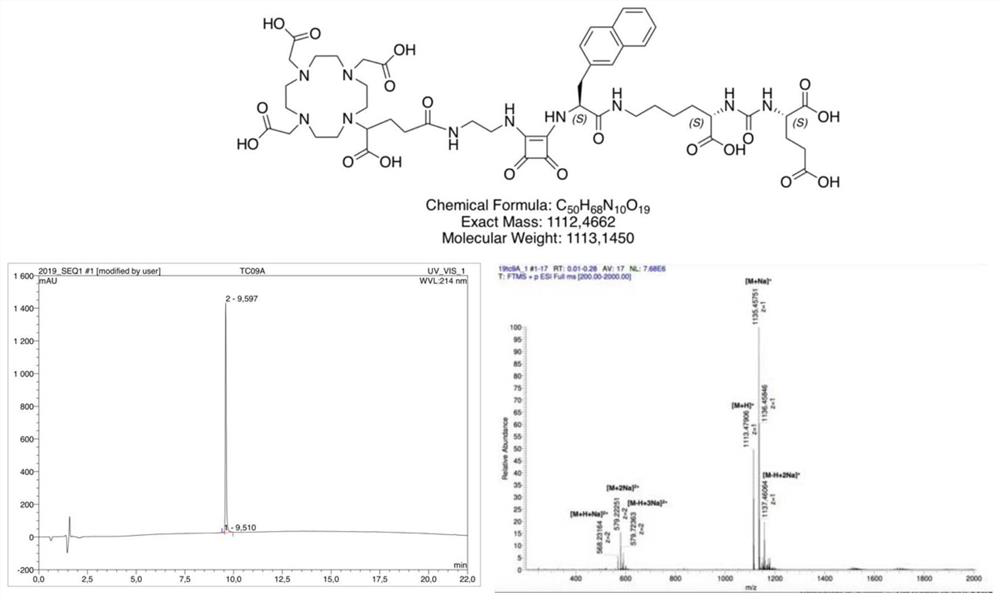
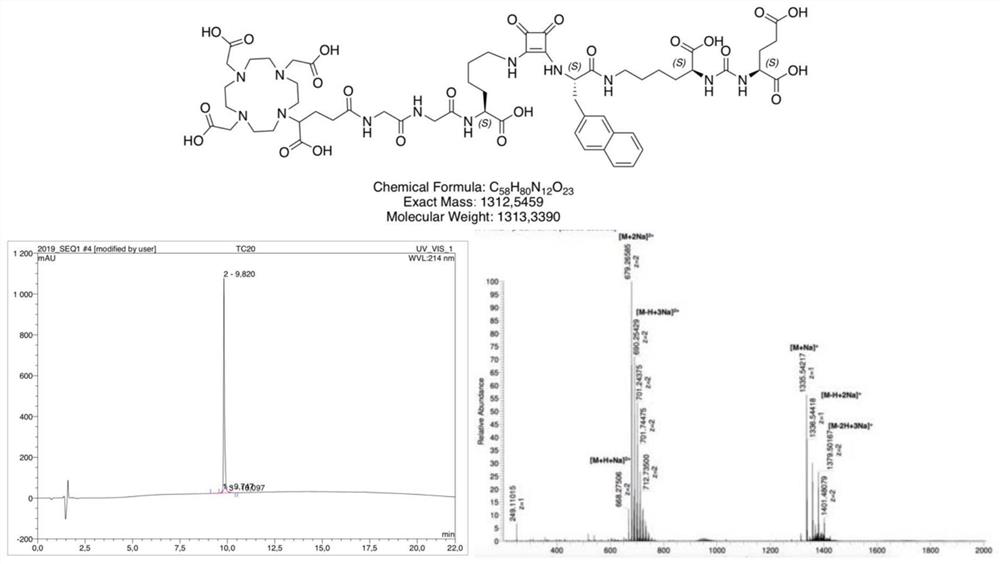
[0148] Example 1: Synthesis of DOTAGA coupled ligands
[0149] DOTAGA-linked PSMA ligands were synthesized by solid-phase peptide synthesis.
[0150] In the first step, by dissolving in 200ml of dry CH at 5°C for 3h 2 Cl 2 3 mmol of bis(tert-butyl)-L-glutamate and 3 ml of N-ethyldiisopropylamine (DIPEA) in 10 ml of dry CH 2 Cl 2 The isocyanate of the glutamyl moiety is generated in situ in a solution of 1mmol of triphosgene in China, and 0.5mmol of resin-immobilized (2-chloro-trityl resin) E-allyloxycarbonyl-protected lysine is added after the reaction And reacted for 16h under gentle stirring, filtered off the resin and used 4ml CH 2 Cl 2 50 mg tetrakis(triphenyl)palladium and 400 μl morpholine in 2 h removed the allyloxy protecting group.
[0151] Subsequent synthesis of the peptidomimetic PSMA-binding motif was performed according to the standard Fmoc protocol, using 2 mmol of the corresponding Fmoc-protected acid, 3.96 nmol of HBTU and 2 mmol of N-ethyl-diisopropylam...
Embodiment 2
[0155] Example 2: 177 Lu radiolabeling and stability testing
[0156] 1nmol of compound 1-5 and PSMA-I&T (purchased from Scintomics GmbH) synthesized in Example 1 were dissolved in 100 μl of 0.5M pH8.0 sodium acetate solution, and 10 μl of 2M pH8.0 sodium acetate solution and 40 μl of 177 LuCl 3 In the mixture of eluent (50MBq), adjust the pH of the marked solution to 4, react at 95°C for 45min, use RP-HPLC and thin-layer chromatography to 177 Lu-labeled compounds were used for quality control, resulting in 77 Lu labeled compounds.
[0157]
[0158] Alternatively, take 30 μl of 177 Lu-labeled compounds were added to 300 μl phosphate buffer saline or human serum, and incubated at 37°C for 1, 6 and 24 hours. Using RP-HPLC and thin-layer chromatography for 177 The stability of Lu-labeled compounds was analyzed. 177 The radiochemical purity and stability data of Lu-labeled compounds are shown in Table 2.
[0159] The results show that the present invention 177 The radi...
Embodiment 3
[0162] Embodiment 3: in vitro PSMA binding test
[0163] Inoculate 5×10 in the right armpit of Balb / c nude mice 5 PSMA high expression (LNCaP C4.2) cells, when the tumor grows to 100-200mm 3 Tumors were excised and 5 mm thick frozen sections were prepared and attached to glass slides. Put the slide into the protein solution, incubate at room temperature for 20min for pre-blocking, then transfer to 40ml containing 0.5% bovine serum albumin phosphate buffer, add 0.2nM of the prepared in Example 2 177 Lu-labeled compound, or add 0.04, 0.2, 1, 5 and 25nM compound of Example 1 at the same time, incubate at room temperature for 1h, then wash the slide with ice-cold buffer solution 4 times for 3min each, air-dry at room temperature and mount on phosphorescence imaging plate and exposed for 15 minutes. Scan slides with a bioimaging analyzer and use image analysis software to calculate 177 Affinity of Lu-labeled compounds to PSMA. The results show that the present invention 177 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com