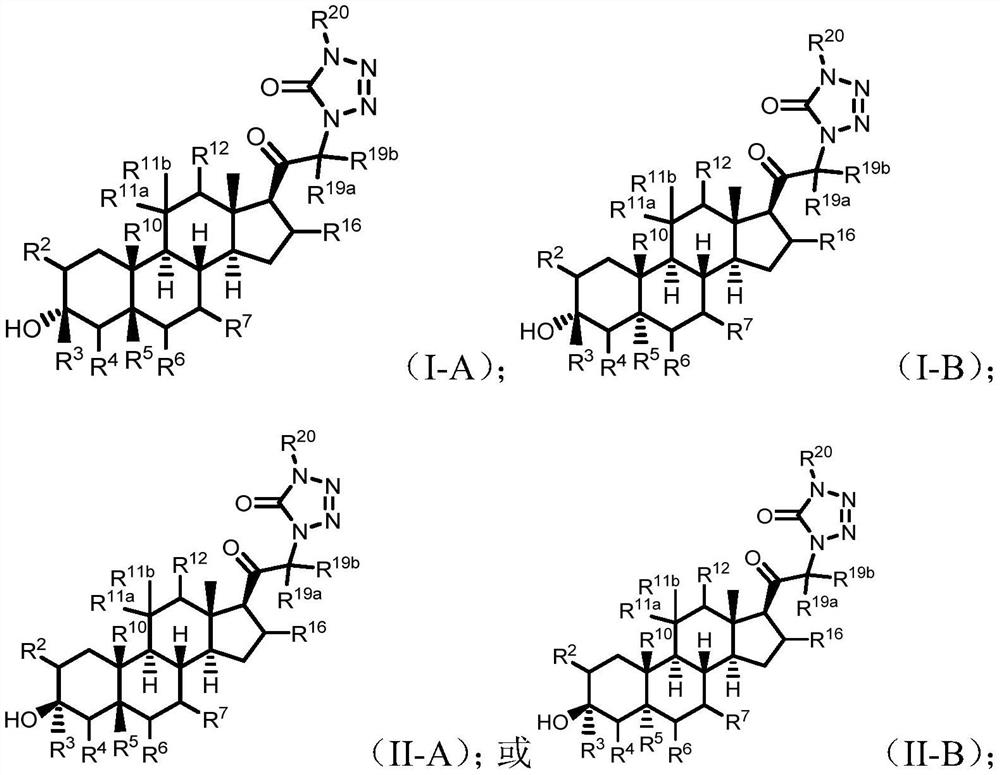
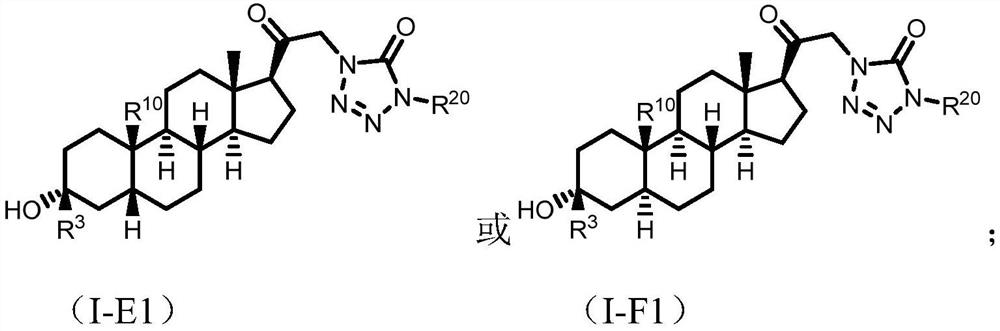
Tetrazolone substituted steroids and use thereof
An unsubstituted, compound technology for use in the field of tetrazolinone-substituted steroids and their uses to solve the problem of not always being effective in treating a desired syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0489] Example 1: 2-bromo-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopentadiene Synthesis of [a]phenanthrene-17-yl)ethan-1-one (compound 7)
[0490] Compound 7 was synthesized according to the procedure disclosed in patent PCT / CN2014 / 075594 and J.Med.Chem.2017,60,7810-7819.
[0491] Step 1: (5R,8R,9R,10S,13S,14S)-13-Methyltetrahydro-3H-cyclopenta[a]phenanthrene-3,17(2H)-dione (compound 2) Synthesis
[0492]
[0493] A mixture of compound 1 (50.0 g, 184.56 mmol, 1.0 eq), palladium black (2.5 g, 5% w / w) in tetrahydrofuran (300 mL) and concentrated hydrobromic acid (1 mL) was hydrogenated with hydrogen at 70 psi. The mixture was stirred at room temperature for 24 hours. The progress of the reaction mixture was monitored by TLC. After the reaction was complete, the mixture was filtered through a pad of celite and a sintered funnel, and the filtrate was concentrated under reduced pressure. The residue was recrystallized from acetone to obt...
Embodiment 2
[0509] Example 2: 1-(2-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxyl-3,13-dimethylhexadecahydro-1H-cyclopentadiene [a] Synthesis of phenanthrene-17-yl)-2-oxoethyl)-4-methyl-1,4-dihydro-5H-tetrazol-5-one (compound 8)
[0510]
[0511] To a suspension of potassium carbonate (42 mg, 0.303 mmol, 0.8 eq) in tetrahydrofuran (5 mL) was added 1-methyl-1,4-dihydro-5H-tetrazol-5-one (114 mg, 1.14 mmol, 3.0 eq ) and compound 7 (150 mg, 0.379 mmol, 1.0 eq). The mixture was stirred at room temperature for 15 hours. The progress of the reaction mixture was monitored by TLC (petroleum ether / ethyl acetate=3:1). After the reaction was complete, the mixture was poured into 10 mL of water and extracted with ethyl acetate (2×20 mL). The combined organic layers were washed with brine (10 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residual mixture was purified by preparative HPLC to provide compound 8 (50 mg, 31%) as a white solid. TLC: PE / EA...
Embodiment 3
[0512] Example 3: 1-ethyl-4-(2-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H- Synthesis of Cyclopenta[a]phenanthrene-17-yl)-2-oxoethyl)-1,4-dihydro-5H-tetrazol-5-one (compound 11)
[0513] Step 1: Synthesis of 1-ethyl-1,4-dihydro-5H-tetrazol-5-one (compound 10)
[0514]
[0515] A stirred mixture of isocyanatoethane (1.0 g, 14.08 mmol, 1.0 eq) and azidotrimethylsilane (3.5 ml, 26.76 mmol, 1.9 eq) was heated to 100 °C and stirred for 16 h. The progress of the reaction mixture was monitored by TLC. After the reaction was complete, the mixture was cooled to room temperature and concentrated. The residue was diluted with ethyl acetate (10 mL) and extracted with saturated aqueous sodium bicarbonate (3 x 10 mL). With effective stirring, 6M hydrochloric acid was added to the combined aqueous layers to adjust pH<3, and extracted with ethyl acetate (3×20 mL). The combined organic layers were dried over anhydrous sodium sulfate and concentrated under redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com